What is the Chemistry of Life?
“As basic building blocks of life, all living organisms use nucleic acids, proteins, lipids, and carbohydrates, as well as a variety of small molecules such as metabolites, messengers, and energy carriers. The study of the structure and function of these biomolecules, as well as their role in biological processes at the molecular, cellular, and organismal levels, is known as the Chemistry of Life.”
Cells are made up of organic and inorganic molecules, which are made up of atoms that have been bonded together.
The following is a simple way to describe the levels of organisation of living things:
atom →molecule→cell→tissue→organ→system→organism→ecosystem
Organic and inorganic compounds must be consumed by living organisms in order for them to be broken down for energy and used as building blocks for the components of life.
Table of Contents
- Atoms and Elements
- Chemical Bonding
- Inorganic Compounds
- Organic Compounds
- Summary
- Frequently Asked Questions – FAQs
Atoms and Elements
Atoms
An atom is the smallest unit of matter that retains all of an element’s chemical properties. Protons, electrons, and neutrons are the three types of subatomic particles found in atoms. An atom is divided into two regions. The first is the atomic nucleus, which is located in the centre of the atom and contains positively charged protons and neutral, uncharged neutrons. The atom’s second, much larger region is a “cloud” of electrons, negatively charged particles that orbit around the nucleus. The atom is held together by the attraction of positively charged protons and negatively charged electrons.
Read more: What Does an Atom Look Like?
Matter
The matter is defined as anything that occupies space and has mass. All matter is composed of substances known as elements. Matter exists in three states.
Elements
Elements are a form of matter that have specific chemical and physical properties and cannot be broken down into other substances via ordinary chemical reactions. There are 118 elements. The four elements that all living organisms share are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N), which account for approximately 96% of the human body.
Properties of Elements include-
Chemical Bonding
All elements do not have enough electrons to fill their outermost shells. The formation of chemical bonds, or interactions between two or more of the same or different elements, are a result of the vacancies in the outermost shells. Atoms will tend to completely fill their outer shells to achieve greater stability and will bond with other elements to accomplish this by sharing electrons, accepting electrons from another atom, or donating electrons to another atom.
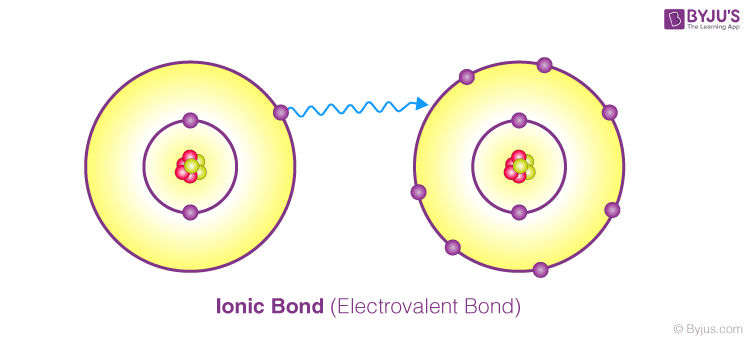
Ionic Bond
A chemical bond is formed between two atoms by donating or accepting one or more electrons from one atom to the other, causing the atoms to achieve their nearest inert gas configuration.
This type of bond is referred to as an ionic bond or an electrovalent bond.
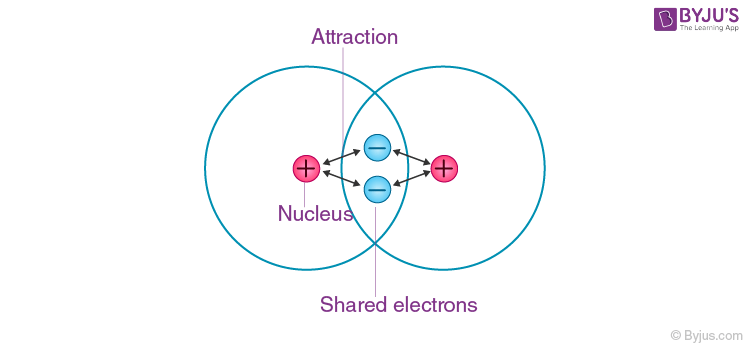
Covalent Bond
A covalent bond is formed when electrons from both participating atoms are shared equally. The pair of electrons involved in this type of bonding is known as a shared pair or bonding pair. Molecular bonds are another name for covalent bonds. The sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell, similar to noble gas atoms.
Inorganic Compounds
Water
Human beings are 72% water. An adult weighing 210 pounds contains approximately 60 litres of water.
Water aids in the maintenance and survival of life. Water transports vital nutrients to all of our cells, particularly muscle cells, delaying muscle fatigue. Water helps with constipation and other abdominal issues, especially for those with IBS. Drinking water or eating foods high in water content can help you lose weight. Toxins are moved through your system more quickly, and kidney function is improved. Inadequate hydration results in impaired kidney function. Water cleanses the body of toxins and waste while also regulating bodily functions such as temperature.

Minerals
Dietary minerals are the chemical elements that all living organisms require in order to function properly. Calcium, phosphorous, potassium, sulphur, sodium, chlorine, and magnesium are examples of essential minerals in humans.
Organic Compounds
Life on Earth would be impossible without carbon. Apart from water, the majority of molecules in living cells are carbon-based and thus are classified as organic compounds. Carbohydrates, lipids, proteins, and nucleic acids are the four major classes of organic compounds. Each of these classes of compounds is made up of large molecules that are made up of small subunits. A monomer is the smallest of these subunits. Polymers are formed when several monomers bond together. Because of the chemical bonds formed, each of these polymers has a distinct structure. These structures are related to the compound’s function in living organisms.
Vitamins
Vitamins are organic compounds that organisms require in limited quantities as vital nutrients. Vitamins are thus necessary for a well-balanced diet. Vitamins perform numerous functions in the body. Some vitamins are coenzymes, which means they help enzymes efficiently catalyse reactions. Some are in charge of metabolism, while others regulate cell and tissue growth and differentiation.

Summary
- Organic and inorganic compounds must be consumed by living organisms in order for them to be broken down for energy and used as building blocks for the components of life.
- Essential compounds are those that a living organism cannot synthesise from other molecules and must obtain from its surroundings.
- Proteins, carbohydrates, and fats are essential organic molecules for living organisms’ growth and survival.
Frequently Asked Questions on Chemistry of Life
What are the five main elements in living organisms?
Oxygen, carbon, hydrogen, nitrogen, and sulphur are all abundant in living organisms (these five elements are known as the bulk elements).
What are the 4 chemical basis of life?
These combine to form the nucleic acids, proteins, carbohydrates, and lipids that are the building blocks of living matter.
What is life made up of?
All living organisms are composed of one or more cells, which are regarded as the basic units of life. Even unicellular organisms are intricate! Inside each cell, atoms combine to form molecules, which then combine to form cell organelles and structures. Similar cells form tissues in multicellular organisms.
What are the different types of chemistry?
The study of matter and how it changes is known as chemistry.
Physical chemistry, analytical chemistry, biochemical chemistry, organic chemistry, and inorganic chemistry are the five major types of chemistry.
What chemicals makeup humans?
Approximately 96% of the mass of the human body is made up of only four elements: oxygen, carbon, hydrogen, and nitrogen, with a large portion of that being water. The remaining 4% is a skewed representation of the periodic table of elements.
Comments