Before learning about condensation polymerization let us quickly recall what polymerization is. Generally, polymerization is defined as a process in which two or more monomers engage in a chemical reaction and form long polymer chains or three-dimensional networks. Polymerization usually occurs via different reaction mechanisms which are affected by factors like the functional groups of the reacting compounds and their non-bonding interactions.
In any case, there are mainly two types of polymerization which are addition polymerization and condensation polymerization. However, we will study the latter on this page.
What is Condensation Polymerization?
It is a form of a step-growth polymerization where smaller molecules or monomers react with each other to form larger structural units (usually polymers) while releasing by-products such as water or methanol molecule. The by-products are normally referred to as condensate.
This polymerization is also sometimes referred to as step-growth polymerization. However, in this context, there are few exceptions that need to be considered. There are instances where molecules of some substances react as condensation polymerization, but they follow the chain growth pattern as seen in additional polymerization.
Characteristics of Condensation Polymerization
Some main characteristics of this type of polymerization are;
- The molecules should have one or two functional groups (like alcohol, amine, or carboxylic acid groups).
- The reaction occurs between two similar or different functional groups or monomers. It can take place between a dimer and oligomer, one monomer and one dimer or between a chain and another chain of polymers.
- Smaller molecules usually combine to form larger molecules.
- Mixed properties of both the molecules or functional groups are taken into consideration.
- A linear polymer is obtained as the condensation product when both functional groups are difunctional.
- When one of the functional groups is tri- or tetra-functional, the polymer formed will be a cross-linked polymer having a three-dimensional network.
- The average molecular weight decreases when monomers are added with one reactive group. Therefore, the functionality of each monomer determines the average molecular weight and cross-link density.
Examples of Condensation Polymerization
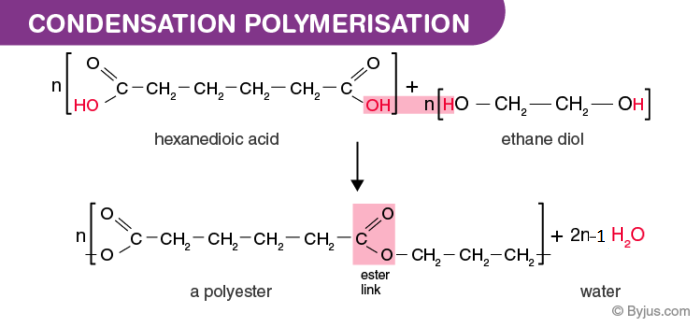
Nylon and Polyester are some of the most common examples of products of this type of polymerization.

HOOC–(CH2)n–COOH + HO–(CH2)m–OH → HOOC–(CH2)n–COO–(CH2)m–OH + H2O
Another form of condensation polymerization is the reaction between a dibasic acid and a glycol where the polymer formed is known as polyester. The by-product again here is water which is mostly removed.


Comments