What is Ethylene?
Ethylene is an unsaturated organic compound with the chemical formula C2H4. It has one double bond and is the simplest member of the alkene class of hydrocarbons.
C2H4 is the simplest alkene with the chemical name Ethylene. It is also called Ethene or Polyethylene or Etileno. It is widely used as a plant hormone, as a refrigerant, and as a food additive.
Ethylene is a colourless gas which has a sweet odour and taste. It is flammable and lighter when compared to air. When exposed to heat or fire for a long duration, the containers can explode.
Table of Content
- Structure of Ethylene
- Properties of Ethylene
- Production of Ethylene
- Chemical Properties of Ethylene
- Uses of Ethylene
- Health Hazards of ethylene
- Frequently Asked Questions-FAQs
Ethylene structure – C2H4
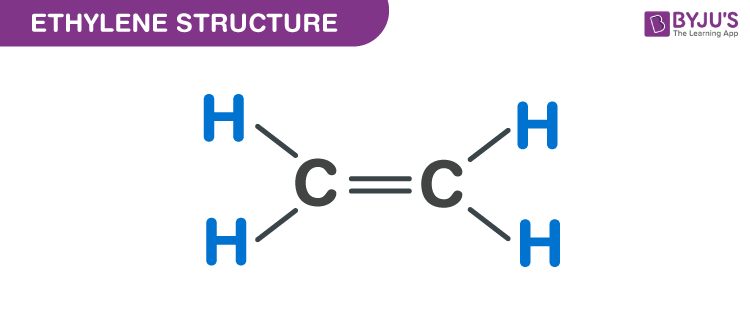
Properties of Ethylene – C2H4
| C2H4 | Ethylene |
| Molecular weight of C2H4 | 28.054 g/mol |
| Density of Ethylene | 1.178 kg/m3 |
| Boiling point of Ethylene | −103.7 °C |
| Melting point of Ethylene | −169.2 °C |
Production of Ethylene
During the year 2013 Etileno was produced by approximately 117 companies from 32 countries.
Petrochemical industry – A predominant method of producing ethylene is steam cracking. Hydrocarbons along with steam are heated to a temperature range of 750–950 °C. It converts large hydrocarbons into smaller hydrocarbons and initiates unsaturation. When feedstock is ethane then the product is ethylene. Polyethylene is separated from the obtained mixture by repetitive compression and the process of distillation. Other methods to produce ethylene include, Fischer-Tropsch synthesis, catalytic dehydrogenation, oxidative coupling of methane, and methanol-to-olefins (MTO).
Laboratory method – It is rarely prepared in the laboratories and is usually purchased. It can be synthesised by dehydrating ethanol with H2SO4 (sulfuric acid) or with aluminium oxide in the gas phase.
Chemical Properties of Ethylene:
(a) Combustion (oxidation with air):
Ethene burns in air or oxygen upon heating to form CO2 and H2O. The combustion reactions are highly exothermic in nature.
C2H4 + 3O2 ⟶ 2CO2 + 2H2O
(b) Polymerization Reactions of ethylene:
Polymerization of ethene leads to polythenes which are of two types:
Low density polyethylene: It is prepared by heating ethene to 463-483 K under the pressure of about 1500 atm. in the presence of traces of oxygen.
n[CH2=CH2] ⟶ [ーCH2ーCH2ー]n
Low density polyethylene is chemically inert, tough and a poor conductor of electricity.
High density polyethylene: It is prepared by the polymerisation of ethene at about 333-343 K under the pressure of 6-7 atm. in a catalyst such as the Ziegler Natta catalyst. This polymer is also inert chemically but is quite tough and hard.
Uses of Ethylene- C2H4
- Ethylene is used in the manufacturing of alcohol.
- Used in the manufacturing of polyethylene.
- Used in promoting senescence.
- Used to produce fabricated plastics.
- Used as a herbicide.
- Used as a curing agent for tobacco.
- Used as a refrigerant.
- Used as an anaesthetic.
- Used to accelerate the ripening of fruits commercially.
- Used in welding metals.
Health Hazards of Ethylene
Average concentration in air can cause drowsiness, unconsciousness, and dizziness. Overexposure may lead to headaches, muscular weakness, and drowsiness. Also, the vapours of this compound can cause asphyxiation. When ethylene is touched in its liquid form, it causes burns, and severe injury. On heating, the fire liberates irritating and toxic gases.
Frequently Asked Questions-FAQs
1. Who discovered ethylene?
A Russian scientist named Dimitry Neljubow demonstrated in 1901, that the active component was ethylene. Doubt discovered that in 1917 ethylene spurred abscission. Not until 1934 did Gane announce that plants synthesise ethylene.
2. How is ethylene produced?
Ethylene is commercially developed by the steam cracking of a wide range of hydrocarbon feedstocks. Processes of olefin cracking and interconversion are being built to improve the efficiency of light olefins. They will usually transform C4-C8 olefins and light gasoline pyrolysis into ethylene and propylene.
3. Is ethylene heavier than air?
Ethylene has the appearance of a colourless gas with a light smell and taste. It is lighter than the atmosphere.
4. Is ethylene polar or nonpolar?
Ethylene is a substance of a nonpolar nature. This is because they have an equal distribution of electrical charges, unlike a polar molecule.
5. What are the functions of ethylene?
In plants, ethylene acts as a hormone. It functions at trace rates during the plant’s life by stimulating or controlling fruit maturation, flower opening and leaves abscission (or shedding).

Comments