What is Galvanic Corrosion?
When two dissimilar metals are in direct contact in a conducting liquid, experience shows that one of the two may corrode. This is called galvanic corrosion.
When two metals having different electrode potentials are in contact with each other in the presence of an electrolyte, one metal acts as a cathode whereas the other acts as an anode. This leads to an electrochemical reaction as a result of which, the anode is dissolved into the electrolyte. This electrochemical process is called galvanic corrosion of metals.
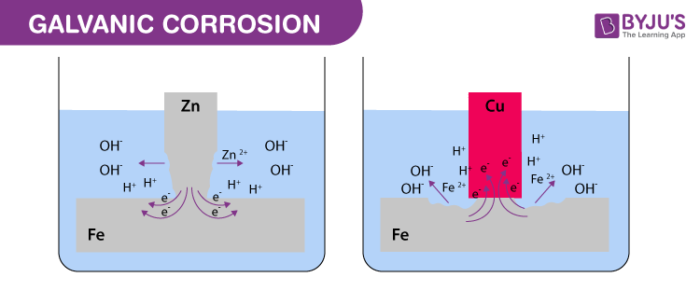
Galvanic corrosion can only occur when there exists an electric conducting path connecting the metals and when an electrolyte which can offer a channel for the migration of ions is present.
Table of Contents
- Recommended Videos
- Examples of Galvanic Corrosion
- Mechanism of Galvanic Corrosion
- Causes of Galvanic Corrosion
- Prevention of Galvanic Corrosion
- Frequently Asked Questions – FAQs
Recommended Videos
Examples of Galvanic Corrosion
The Statue of Liberty in New York, USA was subject to galvanic corrosion. The corrosion had occurred between the support structure made up of wrought iron and the copper exterior of the statue. This led to the rusting of the iron support structure.
Another example of galvanic corrosion occurred in the combat ship ‘USS Independence’. The aluminium hull of the ship had a steel water jet propulsion system attached to it. The aluminium hull acted as an anode towards steel in this corrosion.
Mechanism of Galvanic Corrosion
When two metals come in contact in the presence of an electrolyte, the more reactive metal acts as an anode whereas the less reactive metal acts as a cathode. The electrolyte offers a channel for the movement of particles, which in turn leads to the rapid eroding of the anodic metal.
Galvanic Corrosion can be used beneficially to protect a cathodic metal from corrosion. A good example of this would be the usage of zinc in batteries to promote the corrosion of zinc to create a potential difference.
Causes of Galvanic Corrosion
This type of corrosion occurs when two dissimilar metals are electrically connected and immersed in a conductive solution. The cathode metal stays safe uncorroded, whereas the anode gets corroded. The attack rate on the anode is stimulated when compared to the uncoupled metal rate. For example, if you connect aluminium and carbon steel and immerse them in seawater, the aluminium corrodes quickly and the steel will be protected.
Prevention of Galvanic Corrosion
There are many methods which can be adapted to prevent galvanic corrosion. A few such methods are described below.
- Electroplating process of the metal to form a thin layer of an inert metal on it can also help reduce galvanic corrosion
- Placement of Electrical Insulation between the two Metals to stop the flow of electrons between them and prevent galvanic coupling.
- Usage of a Galvanic isolator which can be two series-semiconductor diodes placed in parallel with two diodes that are conducting in the opposite direction.
- Usage of impermeable coating on the metal surface such as paint can help make sure that the metal won’t come in contact with an electrolyte, which is a good way to prevent galvanic corrosion.
- Using metals with similar potentials reduces the amount of galvanic current that will flow through them. Therefore, this reduces the rate of galvanic corrosion of the metals.
Read more:
Frequently Asked Questions – FAQs
Can galvanic corrosion occur without an electrolyte?
Galvanic corrosion does not occur where all of these conditions are absent. In an electrolyte, galvanic corrosion accelerates the natural corrosion of a metal. Metals may suffer from uniform corrosion, crevice corrosion, pitting, or other types of corrosion, often without galvanic corrosion.
Can galvanic corrosion occur in the air?
Galvanic corrosion (also called bimetallic corrosion) is an electrochemical mechanism in which in the presence of an electrolyte, one metal preferentially corrodes when it is in electrical contact with another. In any electrolyte, including moist air or soil, and in chemical conditions, galvanic cells may form.
What is required for galvanic corrosion?
There must be three conditions for galvanic corrosion to occur: electrochemically dissimilar metals must be present. They must be in electrical contact with these metals, and. It is necessary to expose the metals to an electrolyte.
What happens during galvanic corrosion?
When two dissimilar metals are dissolved in a conductive solution and electrically connected, galvanic corrosion happens. One metal is covered (the cathode), while the other (the anode) is corroded. Compared with the rate when the metal is uncoupled, the attack rate on the anode is accelerated.
How do you protect aluminium from galvanic corrosion?
For aluminium, galvanic corrosion can be easily prevented with a single coat of chrome phosphate pretreatment supplemented by primer and high-performance coating. A single field-applied coat of heavy-bodied bituminous paint may instead be used. For galvanic isolation, anodic coatings alone are normally ineffective.

Comments