Define Solution
A solution is defined as
a homogenous mixture which mainly comprises two components namely solute and solvent.
For example, salt and sugar is a good illustration of a solution. A solution can be categorized into several components.
Read more ⇒ Homogeneous Mixture
Table of Contents
- Different Types of Solutions
- Recommended Videos
- Mixtures
- Homogenous and Heterogeneous Solutions
- Frequently Asked Questions – FAQs
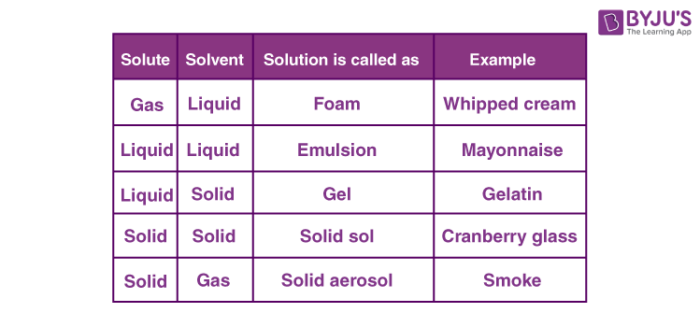
On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions.
In solid solutions, solute and solvent are in the solid-state. For example ceramics and polymer blends. In liquid solutions, solid, gas or liquid is mixed in a liquid state. Gaseous solutions are usually homogenous mixtures of gases like air. Depending upon the number of solutions and solutes, it can be classified into dilute and concentrated solutions.
Different Types of Solutions
Depending upon the dissolution of the solute in the solvent, solutions can be categorized into supersaturated solution, unsaturated and saturated solutions.
- A supersaturated solution comprises a large amount of solute at a temperature wherein it will be reduced, as a result the extra solute will crystallize quickly.
- An unsaturated solution is a solution in which a solvent is capable of dissolving any more solute at a given temperature.
- A saturated solution can be defined as a solution in which a solvent is not capable of dissolving any more solute at a given temperature.
The solutions are of two forms, depending on whether the solvent is water or not.
- Aqueous solution – When a solute is dissolved in water the solution is called an aqueous solution. Eg, salt in water, sugar in water and copper sulfate in water.
- Non-aqueous solution – When a solute is dissolved in a solvent other than water, it is called a non-aqueous solution. Eg, iodine in carbon tetrachloride, sulphur in carbon disulfide, phosphorus in ethyl alcohol.
Solutions are spoken of as having two components, the solvent and the solute. Another classification of the solution depends on the amount of solute added to the solvent.
- A dilute solution contains a small amount of solute in a large amount of solvent.
- A concentrated solution contains a large amount of solute dissolved in a small amount of solvent.
Recommended Videos
Types of Solutions

Most Important Questions From Solutions

Mixtures
A mixture is composed of two or more substances, but they are not chemically combined. In contrast, the compound contains various elements that are bonded to each other. For instance, consider a mixture of salt, that is when salt is dissolved in water it is a mixture but ideally, salts consist of two components namely sodium and chlorine.
Here Sodium and Chlorine are bonded together with the electrostatic force of attraction to form sodium chloride even though there is no chemical bond between water and salt in the mixture. Hence, matter can be classified as mixtures, compounds and elements. Further mixtures can be classified as homogeneous and heterogeneous mixtures.
Read in Detail ⇒ Mixtures
Homogenous and Heterogeneous Solutions
Homogeneous solutions are solutions with uniform composition and properties throughout the solution. For example a cup of coffee, perfume, cough syrup, a solution of salt or sugar in water, etc.
Heterogeneous solutions are solutions with non-uniform composition and properties throughout the solution. A solution of oil and water, water and chalk powder and solution of water and sand, etc.
Examples
Aerated drinks, Salt-water or Sugar water mixtures, and fruit juices are some examples for solutions. Some solutions are heterogeneous in nature, and they are termed as suspension.
Such suspended particles can be seen quite clearly in the solution. Hence, when light is passed through such solutions, it scatters in different directions. Medicated syrups are one of the finest examples of this.
Frequently Asked Questions – FAQs
What is a true solution?
A True Solution is a homogeneous combination of two or more components immersed in a solvent with a particle size of less than 10-9 m or 1 nm. Example: The basic solution of sugar in water. By using filter paper that is often not noticeable to the naked eye, particles cannot be separated from real solutions.
What type of solution is vinegar?
Vinegar, which can contain flavourings, is an aqueous solution of acetic acid and trace chemicals. Usually, vinegar contains 5 to 8 percent acetic acid by amount. The fermentation of ethanol or sugars by acetic acid bacteria normally produces acetic acid.
What is solution and its type?
A solution is a combination of liquid and solute molecules that is homogeneous. Water, concrete or gaseous may be a solution. In comparison, a combination of liquids , gases and solids may be a solution. In certain cases, the solution consists of a number of various kinds of solutes, such as salts, oxygen, and organic compounds, including seawater.
What are liquids? Give two examples.
Examples of liquids at room temperatures include water, mercury, palm oil, and ethanol. While francium, cesium, gallium, and rubidium liquefy at slightly elevated temperatures, Mercury is the only metallic element that is a liquid at room temperature.
What type of solution is formed when two liquids do not mix?
Whether two fluids can be combined to form a solution, they are considered “miscible.” Whether two fluids cannot be mixed to form a solution, they are considered “immiscible.” Alcohol and water are an example of miscible fluids. Oil and water are an example of immiscible liquids.
Read more:

Super byjus helped to me to understand better
Best
Good explained
It helped me in my homework
It help me in my home work ,