We all know that electrons in an atom or a molecule absorb energy and get excited, they jump from a lower energy level to a higher energy level, and they emit radiation when they come back to their original states. This phenomenon accounts for the emission spectrum through hydrogen too, better known as the hydrogen emission spectrum.

In the late 1800s, it was known that when a gas is excited using an electric discharge and the light emitted is viewed through a diffraction grating; the spectrum observed consists of individual well-defined wavelengths instead of a continuous band of light. Experiments have shown that the wavelengths of the lines were characteristic of the chemical element emitting the light. They were an atomic fingerprints which resulted from the internal structure of the atom.
Table of Contents
What is Hydrogen spectrum?
The hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. The hydrogen emission spectrum comprises radiation of discrete frequencies. These series of radiation are named after the scientists who discovered them.
Recommended Videos
Hydrogen Spectrum

Applying Concepts: Spectrum- Definition, Types and More

Hydrogen spectrum wavelength
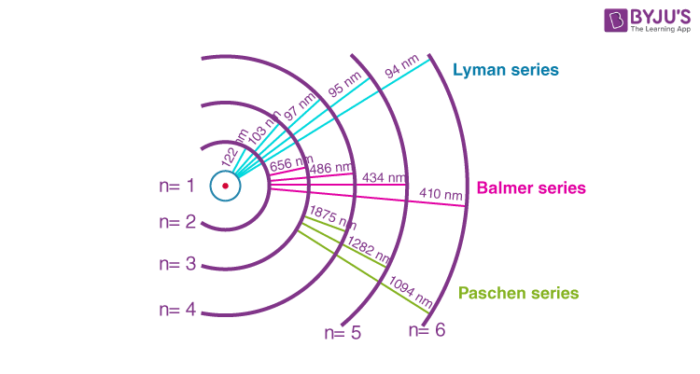
When a hydrogen atom absorbs a photon, it causes the electron to experience a transition to a higher energy level, for example, n = 1, n = 2. When a photon is emitted through a hydrogen atom, the electron undergoes a transition from a higher energy level to a lower, for example, n = 3, n = 2. During this transition from a higher level to a lower level, there is the transmission of light occurs. The quantized energy levels of the atoms, cause the spectrum to comprise wavelengths that reflect the differences in these energy levels. For example, the line at 656 nm corresponds to the transition n = 3 n = 2.
Hydrogen emission spectrum:
In the year 1885, on the basis of experimental observations, Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved. This formula is given as:
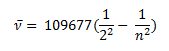
This series of the hydrogen emission spectrum is known as the Balmer series. This is the only series of lines in the electromagnetic spectrum that lies in the visible region. The value, 109,677 cm-1, is called the Rydberg constant for hydrogen. The Balmer series is basically the part of the hydrogen emission spectrum responsible for the excitation of an electron from the second shell to any other shell. Similarly, other transitions also have their own series names. Some of them are listed below,
-
-
-
- The transition from the first shell to any other shell – Lyman series
- The transition from the second shell to any other shell – Balmer series
- The Transition from the third shell to any other shell – Paschen series
- The transition from the fourth shell to any other shell – Brackett series
- The transition from the fifth shell to any other shell – Pfund series
-
-

Johannes Rydberg, a Swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from one orbit to another. The general formula for the hydrogen emission spectrum is given by:
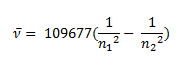
Where,
n1 = 1,2,3,4 …
n2 = n1 +1
ν= wave number of electromagnetic radiation. The value 109,677 cm-1 is known as Rydberg constant for hydrogen.
To learn more about hydrogen emission spectrum download BYJU’S – The Learning App.
Read more:

Fantastic app