Table of Contents
- What is Chromic oxide?
- Properties of Chromic oxide – Cr2O3
- Chromic oxide structure – Cr2O3
- Cr2O3 Uses (Chromic oxide)
- Production of Chromic oxide
- Chemical reactions
- Frequently Asked Questions
What is Chromic oxide?
Cr2O3 is an inorganic compound with the chemical name Chromic oxide. It is also called Dichromium trioxide, or Chromium (3+) oxide, or Chromium (III) oxide. It naturally occurs in a mineral eskolaite, which is mostly found in chromium-rich skarns, tremolite, chlorite veins, and meta quartzites. Chromium (III) oxide appears as a fine light to dark green, hexagonal crystals. It is amphoteric and insoluble in water.
Properties of Chromic oxide – Cr2O3
| Cr2O3 | Chromic oxide |
| Molecular weight of Cr2O3 | 151.9904 g/mol |
| Density of Chromic oxide | 5.22 g/cm3 |
| Boiling point of Chromic oxide | 4,000 °C |
| Melting point of Chromic oxide | 2,435 °C |
Chromic oxide structure – Cr2O3
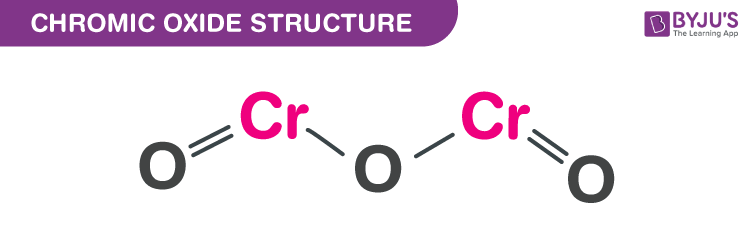
The exact mass and the monoisotopic mass of Chromium (3+) oxide is 151.866 g/mol. The number of hydrogen bond acceptors equals three and the number of hydrogen bond donors equals zero. This compound is canonicalized and has five covalently bonded units.
Cr2O3 Uses (Chromic oxide)
- Chromic oxide is used in electric semiconductors.
- Used in the colouring glass.
- Used in promoting senescence.
- Used in dyeing polymers.
- Used in the manufacturing of chromium metal.
- Used as a catalyst to prepare butadiene.
- Used in stainless steel polishing.
- Used as an anaesthetic.
- Used to add colours in drugs.
- Used in welding metals.
Production of Chromic oxide
In 1838, the Parisians Pannetier and Binet prepared the transparent hydrated form of Chromium (III) oxide. It is obtained from the mineral chromite. The conversion of chromite to chromia is as follows:
Na2Cr2O7 + S → Na2SO4 + Cr2O3
The oxide can also be made by decomposing chromium salts or by exothermically decomposing ammonium dichromate.
(NH4)2Cr2O7 → Cr2O3 + N2 + 4 H2O
Chemical reactions:
Dichromium trioxide when heated with finely divided aluminium or carbon reduces to chromium metal. The reaction is as follows:
Cr2O3 + 2Al → 2Cr + Al2O3
Heating Chromium (III) oxide with carbon and chlorine gives CrCl3 and carbon monoxide (CO):
Cr2O3 + 3Cl2 + 3C → 2CrCl3 + 3CO
Oxidation of chromium (III) oxide results in chromates:
Frequently Asked Questions
Is chromic oxide acidic or basic?
What is chromic oxide used for?
What is the total charge on the Cr2O3 molecule?
The two chromium cations hold a charge of +3 each, amounting to a total of +6. Each oxygen ion holds a charge of -2, totalling to -6. The ionic compound is, therefore, neutral.

Comments