What are polymers?
Polymers are macromolecules which are formed by certain repeating units. These repeating units are called monomers. Monomers have some functional groups which react with each other to form a long polymeric chain. The chemical bonds between monomer units are usually covalent bonds which are formed due to addition reaction between some unsaturated monomer units or condensation reaction between two different functional groups such as -COOH, -NH2, -OH etc.
Table of Contents
- What is polymerisation?
- Recommended Videos
- Polyethylene Terephthalate Definition
- Polyethylene Terephthalate Structure
- How is Polyethylene Terephthalate Made?
- Polyethylene Terephthalate Properties
- Polyethylene Terephthalate Uses
- Frequently Asked Questions – FAQs
What is polymerisation?
The process of formation of polymers from monomer units is called a polymerization. Polymerization occurs because of the addition reaction between unsaturated monomer units or condensation between two different monomers with the elimination of certain small molecules such as water, carbon dioxide, etc.
Recommended Videos
Polyethylene Terephthalate Definition
“Polyethylene terephthalate is a condensation polymer of ethylene glycol and terephthalic acid.” The by-product of the reaction is water so it is an example of condensation or step-growth polymerization.
On the basis of mechanism, there are two types of polymerization; step-growth and chain-growth polymerization. Chain growth polymerization is also called addition polymerization in which monomer units are bonded with each other through their multiple bonds. For example polymerization of ethylene leads to the formation of polyethylene polymer which is also known as polythene.
This polymerization is a three-step process. The first step is initiation which involves the formation of radicals followed by the radical’s reaction with a monomer, the second step is propagation which is the rapid and progressive addition of monomers to the growing polymer chain without a change of the active centre, and the last step is termination which involves the destruction of the growth active centre, usually by combination or coupling of the radicals of two growing polymer chains or by disproportionation. In addition to these three processes, chain transfer might occur, which is the transfer of the growth active site from the active chain to an inactive (dormant) one, a monomer or a solvent molecule (transfer agent).
Polyethylene Terephthalate Structure
In acidic medium, protonation of terephthalic acid occurs that reacts with ethylene glycol to form an intermediate which rearranges and with the transfer of OH- ions, forms polyethene terephthalate polymer.
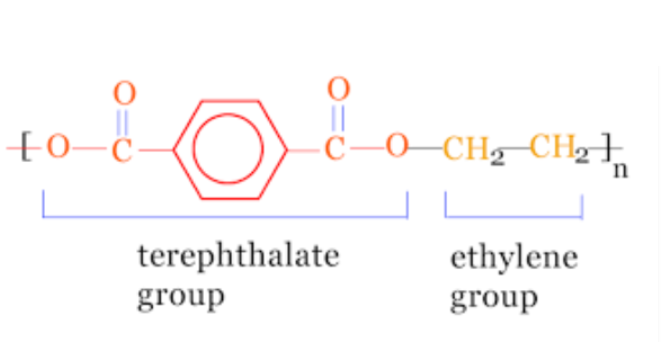
Overall both monomer units are joined together with ester linkage, therefore commonly called polyester. It is a chemical compound that is used to make plastic and fibre. It is formed by polycondensation of terephthalic acid and ethylene glycol.
How is Polyethylene Terephthalate Made?
Polyethylene terephthalate which is also abbreviated as PET / PETE is mainly used to manufacture the packaging material for food products such as fruit and drinks containers. It is lightweight, transparent and also available in some colour. It is a member of the ester family so also called as polyester. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding.
PET / PETE can also be recycled back to its original elements as well as into polyester fibres. These polyester fibres are used in the manufacturing of synthetic carpets, synthetic clothing and other textile products. PET fibres are wrinkle-free and less expensive therefore often mixed with natural fibres. It is also used for the manufacture of microwavable trays and for the packaging of microwavable meals, in containers for cosmetic products and pharmaceutical products.
Polyethylene Terephthalate Properties
- Crystal clear polymer – It is a crystal clear polymer with good purity and health. You must have seen the sparkling PET bottles with brilliant glass-clear presentation attract us.
- Purity – The products of PET taste good and comply with international food contact regulations.
- Safe – The objects made from PET like bottles are tough and virtually unbreakable therefore can be easily used for storage and transportation. This polymer has a high impact and tensile strength that makes it ideal for carbonated products.
- Good barrier – PET products have low permeability to oxygen, carbon dioxide and water, therefore, it maintains the integrity of products with good shelf life.
- Lightweight – The lightweight of PET products reduces the shipping costs compared to glass products.
- No Leakage and damage – Due to the absence of a weld line in the base, PET bottles are free from leakage and damage.
- Recyclable – PET polymer is recyclable and can be reshaped in different shapes.
- Good resistance power – PET products have good resistance against different chemicals such as acids, bases, etc.
Polyethylene Terephthalate Uses
A list of uses of polyethylene terephthalate is given below.
- For the manufacturing of shopping bags, water bottles, videotapes.
- For manufacturing of, containers and bags.
- For the manufacturing of clothes and housing material.
- For manufacturing of water bottles.
- For manufacturing of microwaves containers.
- For the manufacturing of carpets.
- For the manufacturing of packaging films.
Frequently Asked Questions – FAQs
What type of plastic is polyethylene terephthalate?
Polyethylene terephthalate is a thermoplastic synthetic substance, malleable under heat and can be placed into nearly any shape known mostly for its short PET form. It belongs to the polyester materials community. In 1941, in the US the base material was made.
Why is polyethylene terephthalate bad?
It includes terephthalate polyethylene (PET or PETE, or polyester). Harms: PET is known to leach trioxide and phthalates with antimony. All of them are health threats. Although antimony can lead to the production of cancer, skin problems, menstrual and pregnancy problems, endocrine disruptors are phthalates. This is why polyethylene terephthalate is bad.
Is polyethylene terephthalate bad for the environment?
Yes, polyethylene terephthalate is bad for the environment because manufacturing PET resin produces more toxic pollutants than manufacturing glass (nickel, ethylbenzene, ethylene oxide, benzene). Manufacturing a 16 oz. PET bottles emit more than 100 times more toxic air and water pollutants than glass bottles of the same size.
Is polyethylene a plastic?
Yes, polyethylene is plastic. It is the most popular plastic in use today is polyethylene or polythene (abbreviated PE; called IUPAC polyethene or poly(methylene)). It is a linear, man-made, homo-polymer addition, mainly used for packaging purposes (plastic bags, plastic films, geomembranes, bottle containers, etc.)
