Introduction
A chemical reaction occurs when one or more compounds, known as reactants, are changed into one or more distinct substances, known as products. Substances are either chemical components or compounds. The constituent atoms of the reactants are rearranged in a chemical reaction, leading to the formation of various substances as products.
Table of Contents
- Sandmeyer Reaction
- Gattermann Reaction
- Balz-Schiemann Reaction
- Finkelstein Reaction
- Swarts Reaction
- Wurtz Reaction
- Wurtz-Fittig Reaction
- Fittig Reaction
- Friedel-Crafts alkylation Reaction
- Friedel-Crafts acylation reaction
- Reimer-Tiemann Reaction
- Kolbe’s Reaction
- Rosenmund Reduction
- Stephen reaction
- Etard reaction
- Gatterman – Koch reaction
- Clemmensen Reduction
- Wolff Kishner Reduction
- Tollens’ test
- Fehling’s test
- Aldol reaction
- Aldol condensation
- Cross aldol condensation
- Cannizzaro reaction
- Kolbe electrolysis
- Hell-Volhard-Zelinsky (HVZ )reaction
- Gabriel phthalimide synthesis
- Hoffmann bromamide degradation reaction
- Carbylamine reaction
- Hinsberg’s Test
- Coupling Reactions
- Frequently Asked Questions – FAQs
Learn more about:
- Class 12 Chemistry Worksheets
- Class 12 Chemistry Important Questions
- Class 12 Chemistry Important 2 mark Questions
- Class 12 Chemistry Important 3 mark Questions
- Class 12 Chemistry Important 5 mark Questions
- Class 12 Chemistry MCQs
- Class 12 Chemistry Index
- Class 12 Chemistry Practical Syllabus
- Class 12 Chemistry Practical Viva Questions
Here are some important chemical reactions which every student of Class 12 must have a thorough understanding of.
-
Sandmeyer Reaction:
The Sandmeyer reaction is a chemical reaction which is used to synthesize aryl halides from aryl diazonium salts. This reaction is a method for substitution of an aromatic amino group by preparing diazonium salt, that is followed by its displacement and copper salts often catalyze it.

The Br, Cl and CN– nucleophiles can be easily present in the benzene ring of benzene diazonium salt in the presence of Copper ion.
-
Gattermann Reaction:
Bromine and Chlorine can be substituted in the benzene ring by preparing the benzene diazonium salt solution with similar halogen acid present with copper powder. This is the Gattermann Reaction.

-
Balz-Schiemann Reaction:
When arene-diazonium chloride is prepared with fluoroboric acid, arene diazonium fluoroborate is precipitated and decomposes to yield aryl fluoride on heating.

-
Finkelstein Reaction:
In the Finkelstein Reaction, Alkyl iodides are prepared easily by the reaction of alkyl chlorides with Nal in dry acetone.
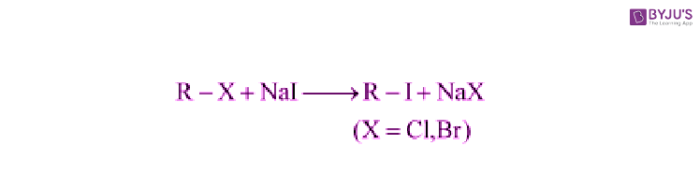
-
Swarts Reaction:
When alkyl chloride is heated in the presence of a metallic fluoride like AgF, Hg2F2, SbF3 or CoF2, we get alkyl fluorides.

-
Wurtz Reaction:
When Alkyl halides react with sodium with dry ether, we get hydrocarbons that include the double number of carbon atoms present in the alkyl halide. This is known as the Wurtz Reaction

-
Wurtz-Fittig Reaction:
When a mixture of alkyl halide and aryl halide gets treated with sodium in dry ether, we get an alkyl arene.

-
Fittig Reaction:
Aryl halides prepared with sodium in dry ether to give analogous compounds where two aryl groups joined.

-
Friedel-Crafts alkylation Reaction:
Benzene is prepared with an alkyl halide in the presence of anhydrous aluminium chloride to give Alkylbenzene.



This is the Friedel-Crafts alkylation Reaction
-
Friedel-Crafts acylation reaction:
We get acyl benzene when an acyl halide is reacted with benzene in the presence of Lewis acids.



Also Read: Acetylation
-
Reimer-Tiemann Reaction:
When preparing phenol with chloroform in the presence of sodium hydroxide, -CHO group is present at the ortho position of the benzene ring, which results into salicylaldehyde” should be replaced with “When phenol is treated with chloroform in the presence of sodium hydroxide, -CHO group is introduced at the ortho position of the benzene ring, which results into the formation of salicylaldehyde.

-
Kolbe’s Reaction:
Phenol reacts with sodium hydroxide to give sodium phenoxide which then reacts with carbon dioxide in acidic medium to give 2-hydroxybenzoic acid (Salicylic acid).

-
Rosenmund Reduction:
Rosenmund reduction is a reaction where acid chlorides are converted into aldehydes by employing hydrogen gas over palladium poisoned by barium sulfate.

-
Stephen reaction:
Nitriles with stannous chloride in the presence of hydrochloric acid are reduced to the corresponding imine and give the corresponding aldehyde after hydrolysis.

-
Etard reaction:
Chromyl chloride oxidizes methyl group present in toluene to get chromium complex which on hydrolysis provides corresponding benzaldehyde.

-
Gatterman – Koch reaction:
Benzene is treated with carbon monoxide and hydrogen chloride in the presence of anhydrous aluminium chloride to give benzaldehyde.

-
Clemmensen Reduction:
In Clemmensen reduction, Carbonyl group of aldehydes and ketones on treatment with zinc-amalgam and concentrated hydrochloric acid reduced to CH2 group.

-
Wolff Kishner Reduction:
Carbonyl group of aldehydes and ketones on treatment with hydrazine which on heating with sodium hydroxide in a high boiling solvent (ethylene glycol) reduced to CH2 group.

-
Tollens’ test:
Heating an aldehyde with fresh prepared ammoniacal silver nitrate solution produces a bright silver mirror due to the formation of silver metal.

-
Fehling’s test:
Fehling’s solution A (aqueous copper sulfate) and Fehling solution B (alkaline sodium potassium tartrate) are mixed in equal amounts before the test. A reddish brown precipitate is obtained when an aldehyde is heated with Fehling’s reagent.

-
Aldol reaction:
Aldehydes and ketones having one α-hydrogen undergo a reaction in the presence of dilute alkali as the catalyst to produce β-hydroxy aldehydes or β-hydroxy ketones.
-
Aldol condensation:
Aldol and Ketol lose water to provide α,β-unsaturated carbonyl compounds which are aldol condensation products.

-
Cross aldol condensation:
Aldol condensation is carried out between two different aldehydes and ketones. It gives a mixture of four products if both of them include α-hydrogen atoms.

-
Cannizzaro reaction:
Aldehydes without α-hydrogen atom undergo self-oxidation and reduction reaction when treated with concentrated alkali.

-
Kolbe electrolysis:
in Kolbe electrolysis, An aqueous solution of sodium or potassium salt of a carboxylic acid gives alkane containing an even number of carbon atoms on electrolysis.

-
Hell-Volhard-Zelinsky (HVZ )reaction:
Carboxylic acids having a α-hydrogen are halogenated at the α-position giving α-halo carboxylic acids on treatment with chlorine or bromine in the presence of small amount of red phosphorus.

-
Gabriel phthalimide synthesis:
Phthalimide prepared with ethanolic potassium hydroxide produces potassium salt of phthalimide when heated with alkyl halide followed by alkaline hydrolysis forms the corresponding primary amine.

-
Hoffmann bromamide degradation reaction:
An amide with bromine in an aqueous solution of sodium hydroxide produces primary amines. Migration of an alkyl or aryl group takes place from carbonyl carbon of the amide to the nitrogen atom. The amine so produced include one carbon less than that present in the amide.

-
Carbylamine reaction:
Aliphatic and aromatic primary amines when heated with chloroform and ethanolic potassium hydroxide produces isocyanides or carbyl amines which are foul smelling substances.
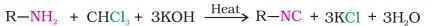
-
Hinsberg’s Test:
Benzenesulfonyl chloride (C6H5SO2Cl) reacts with primary and secondary amines to produce sulphonamides.
- The reaction of benzene-sulfonyl chloride with primary amine yields N-ethyl benzene-sulfonyl amide. The hydrogen attached to the nitrogen in sulphonamide is strongly acidic due to the presence of strong electron withdrawing sulfonyl group. Hence, it is soluble in alkali.

- In the reaction with a secondary amine, N, N-diethyl- benzenesulfonamide is formed. Since N, N- diethyl benzene sulphonamide does not contain any hydrogen atom attached to a nitrogen atom, it is not acidic and hence insoluble in alkali.

- Tertiary amines do not react with benzene-sulfonyl chloride.
- The reaction of benzene-sulfonyl chloride with primary amine yields N-ethyl benzene-sulfonyl amide. The hydrogen attached to the nitrogen in sulphonamide is strongly acidic due to the presence of strong electron withdrawing sulfonyl group. Hence, it is soluble in alkali.
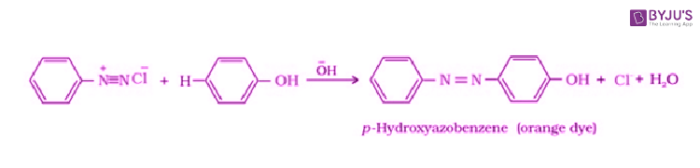
Coupling Reactions:
Benzene diazonium chloride gets reacted with phenol in which the phenol molecule at its para position is mixed with the diazonium salt to give p-hydroxyazobenzene.
Recommended Videos
Most Expected Named Reactions

To know more about Chemical Reactions in detail, register with BYJU’S.
Frequently Asked Questions – FAQs
What is a Sandmeyer Reaction?
The Sandmeyer reaction is a flexible synthetic tool by which an amino group on an aromatic ring is replaced with a broad range of substituents by converting an amino group attached to an aromatic ring into a diazonium salt that can be converted into several functional groups.
What is a Finkelstein Reaction?
The Finkelstein reaction is an organic process in which an alkyl halide is exchanged for another alkyl halide via a metal halide salt reaction. This reaction occurs in an equilibrium phase, taking advantage of the low solubility of acetone in freshly produced metal halide salts.
What is Wurtz reaction example?
Wurtz reactions are used to produce ethane from methyl chloride. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether.
What is product of Kolbe reaction?
Ortho-hydroxybenzoic acid (salicylic acid) is formed as the primary product.
Why Sulphur is used in Rosenmund reaction?
In the Rosenmund reaction, sulphur is used to prevent the aldehyde from hydrogenating further.

It’s good for students
Was really helpful for my exams. thank you!
This link is best for me thanks byjus team
This link is best for me thanks byjus team
It is very useful for everyone
It’s very good !!!
Thank u for ur information
it is useful for all students
It is really helpful for us
thank you it’s really useful for us
It is very useful, thank you and keep it up👍🏻
Good job byjus love you#.
Thanks It is very help full for all students
Thanku byjus sereously it is really help full for me 👍👍
Nice and easy explanation sir tqqq
Thanks anlot sir🙏🙏🙏🙏
thank u very much to gave me a lot of information about reactions
In the pandemic situation its really helpful for students for boards tq byju’s team
Thank you very much byju’s team! I can revised all reactions very fast. Here also available previous year questions and it is helpful to all JEE aspirants. THANK YOU!
Thank you so much. It will be very useful for my exams.
This link is very useful to me for my exam thank you
Thankyou so much it is a very good and best material for quick reading
thanks byju’s team for providing this important reaction
very impressive and precise notes