What is Zinc Sulfate (ZnSO4)?
ZnSO4 is an inorganic compound with the chemical name Zinc Sulfate.
Zinc Sulfate is a dietary supplement. It was historically called white vitriol. It is also known as Zincate, Zinc sulfate (1:1). This compound is listed on the World Health Organization’s List of Essential Medicines. You must avoid taking this medication with foods that are high in phosphorus or calcium because it can make it harder for your body to absorb.
Zinc Sulfate is odourless and has a white powder appearance. Zinc Sulfate is non-combustible and soluble in water. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. It is widely used in the prevention and treatment of zinc deficiency.
Table of Contents
Properties of Zinc Sulfate – ZnSO4
| ZnSO4 | Zinc Sulfate |
| Molecular Weight/ Molar Mass | 161.47 g/mol |
| Density | 3.54 g/cm³ |
| Boiling Point | 740 °C |
| Melting Point | 680 °C |
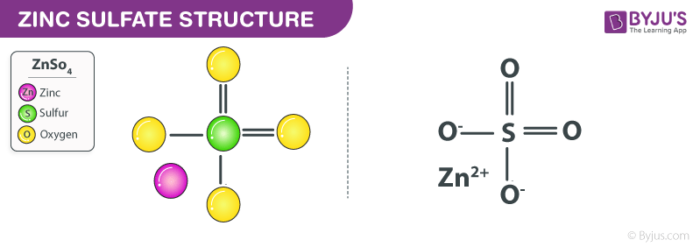
Zinc Sulfate Structure (ZnSO4 Structure)
Zinc Sulfate (ZnSO4 ) Uses
- Medically it is used along with oral rehydration therapy.
- It acts as a coagulant in the production of rayon.
- It is used as a preservative for leathers.
- It is used in zinc electroplating as an electrolyte.
- It is used as a mordant in dyeing.
- It is used in the brewing process as a zinc supplement.
- It is used as an astringent in eye drops and lotions.
- It is used to treat acne.
- You can take zinc sulfate with food if you are suffering from stomach upset.
Frequently Asked Questions
What is zinc sulfate used for?
Zinc is a mineral that occurs naturally. Zinc is important for growth, and for body tissue development and health. Zinc sulfate is used to treat zinc shortages and avoid them.
Is zinc sulphate dangerous?
Zinc sulfate breathing can irritate the respiratory tract, cause nausea, vomiting, stomach ache, dizziness, depression in the mouth, metallic taste and death. Skin contact exposure can damage the skin which leads to ulcers, blisters and scarring.
How much zinc is in zinc sulfate monohydrate?
Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. This quantity is usually stated on your supplement label, making it easy to decide how much you will take to meet your daily needs.
What is the best form of zinc to take as a supplement?
Zinc picolinate, zinc acetate, and zinc citrate are the three types of zinc supplements which the body most easily absorbs. It’s smart to have an abundance of vitamins at hand to boost the immune system when the cold and flu season arrives.
What is the difference between zinc and zinc sulfate?
Zinc sulfate is a water-soluble form, meaning it is absorbed readily by the body. In OTC lozenges this chelated form is also used to avoid colds or to treat deficiencies in zinc. A further type of chelated zinc is zinc gluconate. This is commonly found in sprays of nasal zinc and is often called Orazinc.
Other related links:
| Calcium | Allotropes of Phosphorus |

Comments