Table of Contents
- Fermentation and its types
- Alcoholic fermentation definition and occurrence
- Agent of Alcoholic fermentation
- Equation for Alcoholic fermentation
- Products of Alcoholic fermentation
- More sub-products of Alcoholic fermentation
- Why fermentation reduces towards the end?
Download Complete Chapter Notes of Microbes in Human Welfare
Download Now
Fermentation
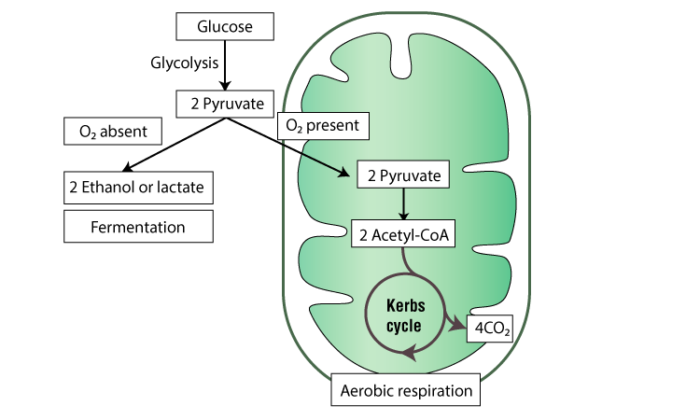
Fermentation is where microorganisms produce a beneficial and desirable change in food. The process of fermentation is commonly deployed in the alcohol industry. Alcoholic fermentation or ethanol fermentation is a biological method wherein the sugar gets transformed into carbon dioxide and alcohol.
Oxygen is not a prerequisite, hence is an anaerobic process and is typically carried out by yeasts. The by-products of this process of fermentation are alcohol, carbon dioxide, water and heat. The process of ethanol fermentation has been in use for millennia.
The process of fermentation occurs in anaerobic conditions, the absence of oxygen and in the presence of beneficial microbes that gain energy through fermentation. Even when there is sufficient oxygen, if there is enough sugar, some of the yeast cells (Saccharomyces cerevisiae) opt for fermentation over aerobic respiration.
The process of fermentation results in microbes disintegrating sugars into alcohols and acids, thus making food nutritious and increasing its shelf life. The products of fermentation render enzymes required for digestion. Food that is fermented comprises enzymes needed to break it down.
Types of Fermentation
Fermentation used is of three different types –
- Lactic acid fermentation
Bacteria and strains of yeast convert sugars or starch into lactic acid and do not require heat. Lactic acid fermentation takes place in human muscle cells too. When a strenuous activity is performed, muscles tend to expend ATP at a faster pace compared to the supply of oxygen to muscle cells. This leads to the accumulation of lactic acid, and hence the sore muscles.
In this event, glycolysis produces ATP, which disintegrates glucose molecules into 2 pyruvate molecules in anaerobic conditions.
- Alcohol fermentation/Ethanol fermentation
In the process, yeasts break molecules of pyruvate, leading to the metabolism of glucose referred to as glycolysis into sugars or starch down to molecules of carbon dioxide and alcohol. Alcoholic fermentation produces beer and wine.
- Acetic acid fermentation
Sugars and starches from fruits and grains ferment into condiments and sour tasting vinegar.
In this article, we discuss alcohol fermentation in detail.
What is Alcoholic Fermentation? – Definition of Alcoholic Fermentation
Alcoholic fermentation is the anaerobic transformation of fructose and glucose (sugars) into ethanol and carbon dioxide. The process is conducted by yeasts and a few bacteria (Zymomonas mobilis).
The process of alcoholic fermentation regenerates the NAD+ taken up at the time of glycolysis, and provides yeast with an energy gain of 2 ATP molecules through the metabolized hexose.
When grape juice is fermented, Saccharomyces cerevisiae (Species of yeast), primarily directs the pyruvate for the production of ethanol to regenerate NAD + consumed by the process of glycolysis. This phenomenon is referred to as alcoholic fermentation.
Steps of Alcoholic Fermentation
The process of alcoholic fermentation can broadly be divided into two main parts –
- Glycolysis – glucose is broken down into 2 pyruvate molecules
- Fermentation – pyruvate molecules are converted into 2 molecules of carbon dioxide and 2 ethanol molecules
Where does Alcoholic Fermentation Occur?
The process of alcoholic fermentation occurs within the cytoplasm.
Agent of Alcoholic Fermentation
It is a well-known fact that the most widely used agent for the process of alcoholic fermentation is S. cerevisiae. This yeast is commonly used as a microbial starter in different fermentation industries.
At the time of alcoholic fermentation of fruits and juices, the S. cerevisiae becomes a dominant species as a result of their strong selective environment, given the low pH and high ethanol and sugar concentrations with anaerobic conditions.
Equation for Alcoholic Fermentation – Alcoholic Fermentation Equation
The reaction occurring in alcoholic fermentation can be summarised as follows –

The process of alcoholic fermentation converts one mole of glucose into 2 moles of ethanol and 2 moles of carbon dioxide, thus producing 2 moles of ATP in the process.
The process of alcoholic fermentation is a complex process. As the reaction proceeds, various biochemical, physicochemical, chemical processes occur, hence turning grape juice into vine.
Products of Alcoholic fermentation
Initially, pyruvate is decarboxylated into ethanal by pyruvate decarboxylase. The enzyme requires cofactors in the form of magnesium and thiamine pyrophosphate. Hence, alcohol dehydrogenase reduces ethanal to ethanol, thus recycling NADH to NAD +.
In Saccharomyces cerevisiae, there are three isoenzymes of alcohol dehydrogenase, however, isoenzyme I is mainly involved in the conversion of ethanal to ethanol. Zinc is used as a cofactor by Alcohol dehydrogenase.
The final products of alcoholic fermentation are ethanol and carbon dioxide. Both are transported to the exterior of the cell by the process of simple diffusion. Apart from ethanol, some other compounds are generated all through the process of alcoholic fermentation, such as esters, higher alcohols, succinic acid, glycerol, 2,3-butanediol, diacetyl, acetoin.
In this process, the NADH donates their electrons to pyruvate’s derivative, thus producing ethanol.
Conversion from pyruvate into ethanol occurs in two steps –
- Production of acetaldehyde – carboxyl group eliminated from pyruvate and released as carbon dioxide, hence producing acetaldehyde, a two-carbon molecule
- NADH passes their electrons to acetaldehyde, thus regenerating NAD+ thus forms ethanol
The alcoholic fermentation by yeasts generates ethanol seen in alcoholic beverages.
More Sub-products of Alcoholic Fermentation
As aforementioned, in addition to mainly producing ethanol and glycerol, some other substances too are produced as a result of fermentation, which is the result of their complex process.
Some of the sub-products of alcoholic fermentation are –
- Acetic acid
- Diacetyl, acetoin and 2,3-butanediol
- Ethanal/acetaldehyde
- Esters
- Higher alcohols
- Succinic acid
Why Fermentation Reduces Towards the End?
At times the process of alcoholic fermentation slows down as it reaches the end. The yeasts dramatically decrease their consumption of sugar and fermentation can cease much before the fermentable sugars are completely metabolised. In this event, there could occur two scenarios –
- Wine is incomplete, something must be done to complete it
- High risk of bacterial spoilage
Some causes of sluggish fermentation are –
- Extremities of temperature
- Complete anaerobiosis
- Presence of medium-chain fatty acids
- Extreme levels of sugar concentrations
- Presence of antifungal substances
- Antagonism between microorganisms
- Nutrient deficiencies
These causes, usually a synergistic combination of a few of these causes, can restrict the correct development of alcoholic fermentation. If the process of fermentation stops finally, the yeast should be reinoculated
Hence, alcoholic fermentation is a complex process involving the transformation of sugars into ethanol and other subproducts.
Glycolysis
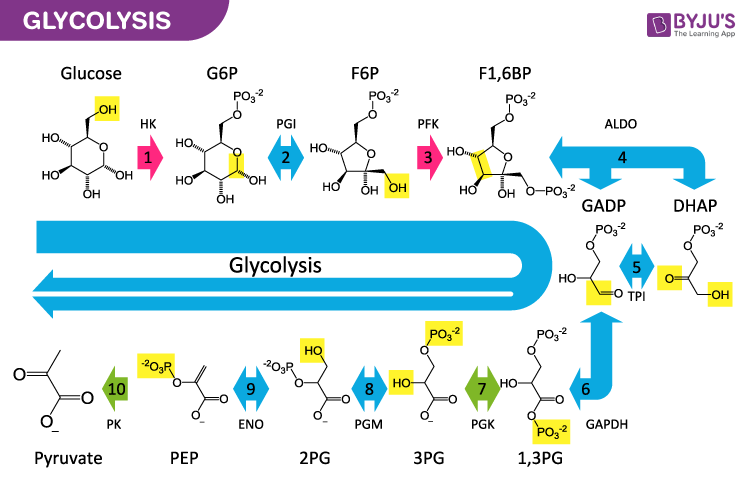
Glycolysis is the metabolic process that converts glucose (C6H12O6) into pyruvic acid (CH3COCOOH). The pathway does not need oxygen, the energy released is used as ATP and reduced NADH. The process of glycolysis, on the whole, is a series of 10 reactions involving the action of enzymes.
Glycolysis mostly takes place in the liquid part of cells (cytosol). The commonly occurring glycolysis is the EMP (Embden–Meyerhof–Parnas) pathway.
Glycolysis and Alcoholic Fermentation
When there is a dearth of oxygen supply during prolonged and heavy exercises, the muscles derive their energy from glycolysis. Under anaerobic conditions, yeasts gain their energy from a similar process referred to as alcoholic fermentation.
In glycolysis, there is a chemical breakdown of glucose into lactic acid, hence making energy available for cellular activity in the form of ATP. Except for the final phase, alcoholic fermentation is just like the process of glycolysis. The pyruvic acid in alcoholic fermentation is disintegrated into carbon dioxide and ethanol. The lactic acid gained from the process of glycolysis leaves a sense of fatigueness, while the products of alcoholic fermentation have been used in brewing and baking for long.
Glycolysis and alcoholic fermentation both are anaerobic processes that start with glucose. This process of glycolysis necessitates 11 enzymes that transform glucose to lactic acid. For the initial 10 steps, the process of alcoholic fermentation follows the same enzymatic route. The lactate dehydrogenase, the last enzyme of glycolysis, is substituted by two enzymes in the process of alcoholic fermentation. Alcoholic dehydrogenase and Pyruvate decarboxylase, both these enzymes convert pyruvic acid into carbon dioxide and ethanol in the process of alcoholic fermentation.
Hence, neither the process of alcoholic fermentation nor glycolysis recognises any gain in energy (ATP) till the tenth enzymatic disintegration.
This was about alcoholic fermentation, its definition, equation, products and subproducts. For related articles on NEET preparation, visit us at NEET BYJU’S.
Also see:
Comments