Flashcards for NEET Chemistry are designed to boost your NEET preparation. Find below flashcards for the chapter “Structure of Atom”. These flashcards are prepared as per the NEET syllabus. These are helpful for aspirants of NEET and other exams during last-minute revision. It covers all the important points that are frequently asked in the exam. Check BYJU’S for the full set of Flashcards and Study material for NEET Chemistry.
Download PDF of NEET Chemistry Flashcards for Structure of Atom
|
Name of the NEET Sub-section |
Topic |
Flashcards Helpful for |
|
Chemistry |
Structure of Atom |
NEET exams |
| Structure of Atom | |
|
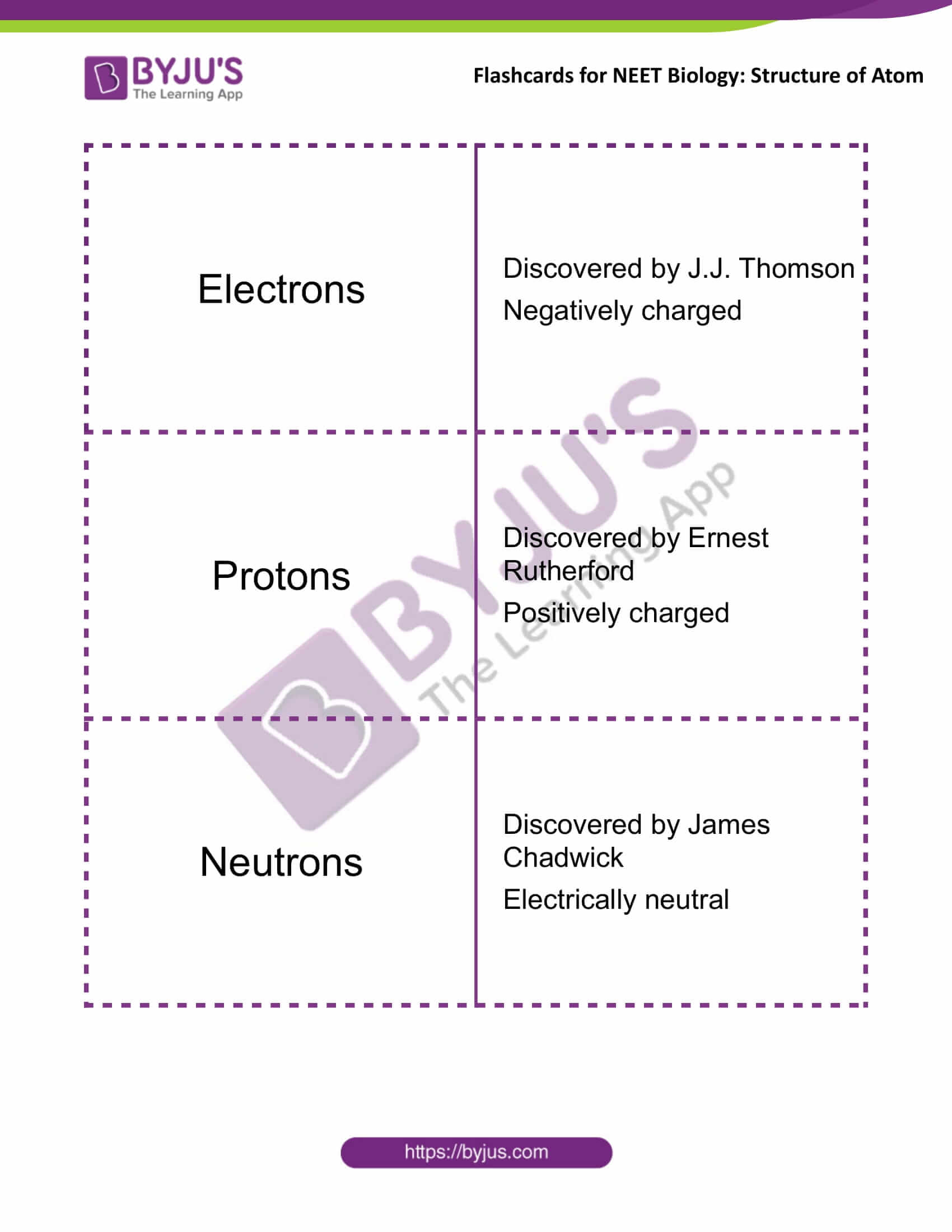
Electrons |
Discovered by J.J. Thomson
Negatively charged |
|
Protons |
Discovered by Ernest Rutherford
Positively charged |
|
Neutrons |
Discovered by James Chadwick
Electrically neutral |
|
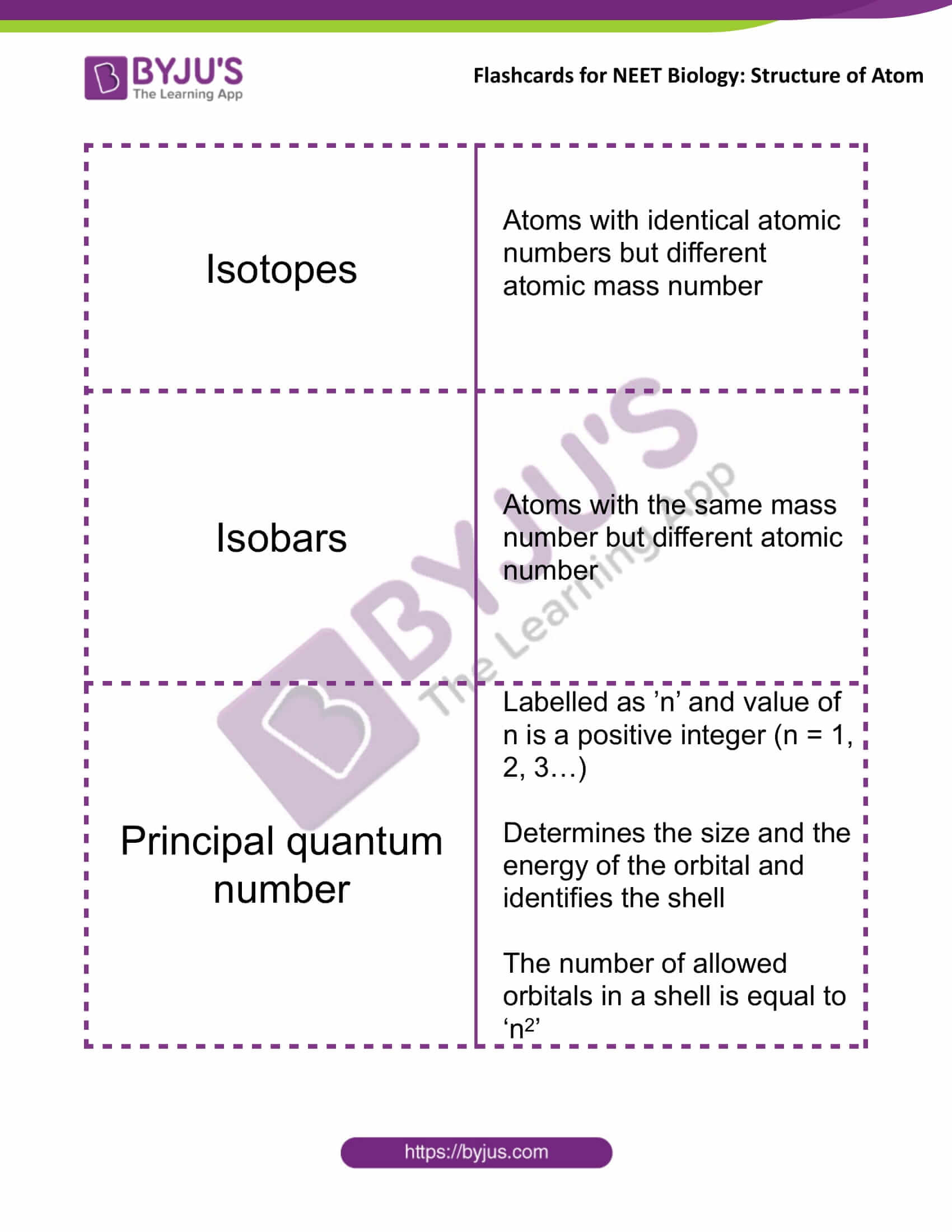
Isotopes |
Atoms with identical atomic numbers but different
atomic mass number |
|
Isobars |
Atoms with the same mass
number but different atomic number |
|
Principal Quantum Number |
Labelled as ’n’ and the value of n is a positive integer (n = 1, 2, 3…)
Determines the size and the energy of the orbital and identifies the shell The number of allowed orbitals in a shell is equal to ‘n2’ |
|
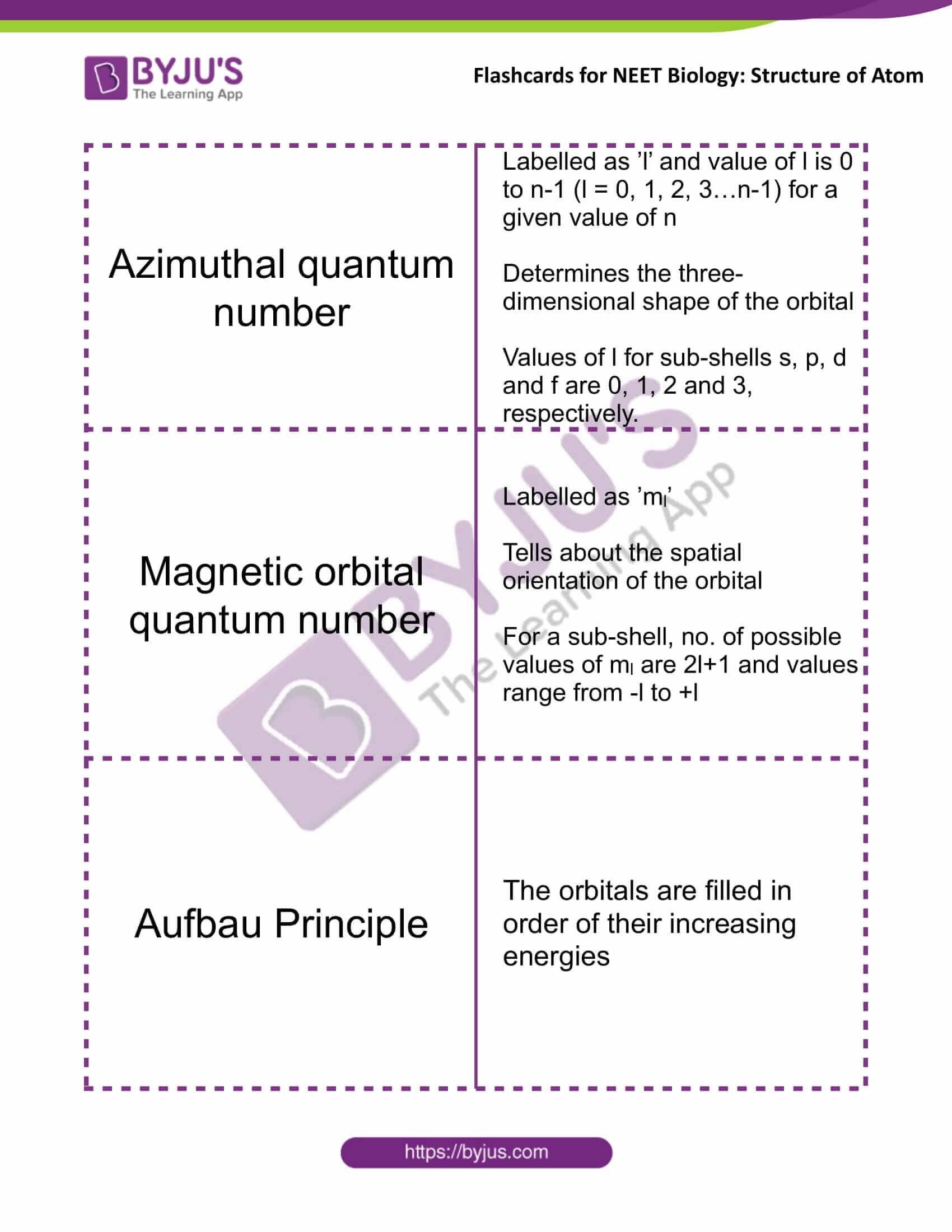
Azimuthal Quantum Number |
Labelled as ’l’ and value of l is 0 to n-1 (l = 0, 1, 2, 3…n-1) for a given value of n
Determines the three-dimensional shape of the orbital Values of l for subshells s, p, d and f are 0, 1, 2 and 3, respectively |
|
Magnetic Orbital Quantum Number |
Labelled as ’ml’
Tells about the spatial orientation of the orbital For a subshell, no. of possible values of ml are 2l+1 and values range from -l to +l |
|
Aufbau Principle |
The orbitals are filled in order of their increasing energies |
|
Pauli Exclusion Principle |
No two electrons in an atom can have the same set of four quantum numbers
Two electrons present in the same orbital have opposite spins |
Get access to the full set of flashcards for NEET Chemistry, only at BYJU’S.
Recommended Video:




| Also Check: |

Comments