The d block elements are sometimes called the transition elements. In the d block elements the elements show considerable similarities to one another even as we move across a period. There are four main facts of the chemistry of d block elements, their redox chemistry, the colours of their compounds, their catalytic activity and their ability to form complexes – compounds in which the metal ion is surrounded by other atoms or ions.
General Trends
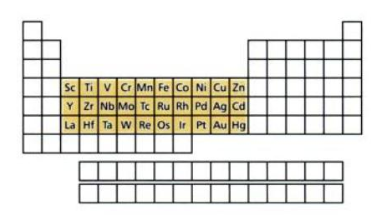
The properties of the d block elements are clearly those of metals. With the exception of mercury, they are lustrous, malleable, ductile and good conductors of heat and electricity.
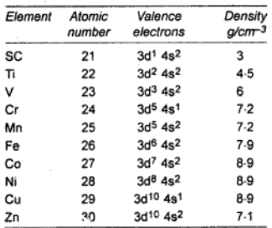
In general, they have higher densities, melting points and boiling points and are harder than the metals of groups 1 and 2.
Density
The variation in metal radii causes the densities of the transition elements to first increase and then decrease across a period. Although the overall change in radii among these elements is small the effect is magnified because the volume is actually changing with the cube of the radius.
Melting and Boiling Points
The transition elements are metals. They have high densities and melting points and are strong and hard. They form compounds of colour, which serve as catalysts.
d Block Elements Oxidation State
Oxidation state is a fundamental concept in chemistry, and is particularly important in transition metal chemistry, as d-block elements often have a wide range of stable oxidation states.
d-Block elements exhibit a variety of oxidation states in their compounds because both ns and (n – 1)d electrons are available for bonding. In each case, the minimum oxidation state is given by the number of outer s-electrons and maximum oxidation state is given by the sum of s and d-electrons.
Standard Electrode Potential
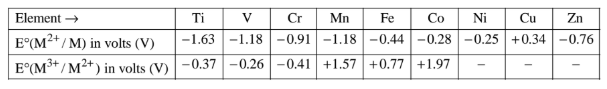
Except for copper and mercury, the standard electrode potential of d-block elements in acid solution is generally negative, that is lower than that of hydrogen. However, copper and mercury are having positive electrode potentials. All the d-block elements with negative reduction potential liberate hydrogen from dilute acid.
The lower the electrode potential that is more negative the standard reduction potential of the electrode, the more stable is the oxidation state of the transition metals ion in the aqueous medium.
Coloured Compounds Formation
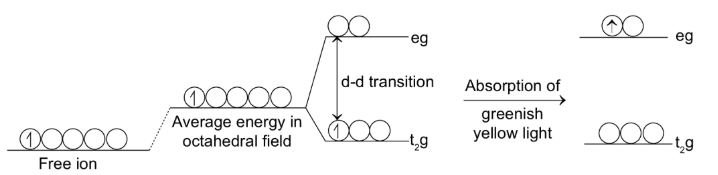
The majority of transition metal ions are coloured. The colour depends on the number of unpaired d-electrons which absorb a portion of white light falling on them.
d Block Elements Compounds
Potassium Permanganate – KMnO4
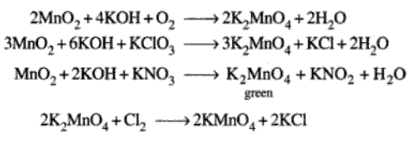
Potassium Dichromate – K2Cr2O7
Potassium dichromate is prepared from chromite ore. The various steps involved are given in the flow sheet diagram below.
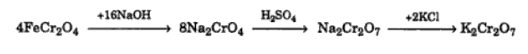
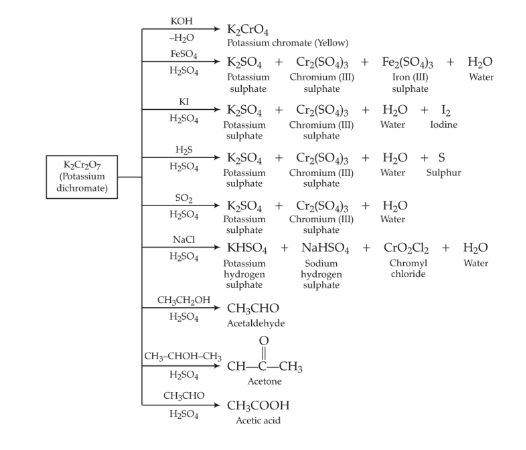
The d-block elements lie between the elements s- and p-block, i.e. these elements are located in the centre of the periodic table. The d-block contains each of ten elements from three sequences. These series are characterized by the fully filled subshells 3d, 4d, and 5d.
Must read:
- NEET Chemistry Syllabus
- How to Score 160 Plus in NEET Chemistry
- NEET Chemistry Weightage
- Chemistry Formulas for NEET
- NEET Chemistry MCQs
- NEET Chemistry Important Topics
VERYYYY NICEEE. THANKS FOR HELPING ME IN NEET EXAMINATION.