Flashcards for NEET Chemistry are designed to boost your NEET preparation. Find below flashcards for the chapter “Hydrocarbons”. These flashcards are prepared as per the NEET syllabus. These are helpful for aspirants of NEET and other exams, during last-minute revision. It covers all the important points that are frequently asked in the exam. Check BYJU’S for the full set of Flashcards and Study material for NEET Chemistry.
Download PDF of NEET Chemistry Flashcards for Hydrocarbons
|
Name of the NEET Sub-section |
Topic |
Flashcards Helpful for |
|
Chemistry |
Hydrocarbons |
NEET Exams |
|
Hydrocarbons |
|
|
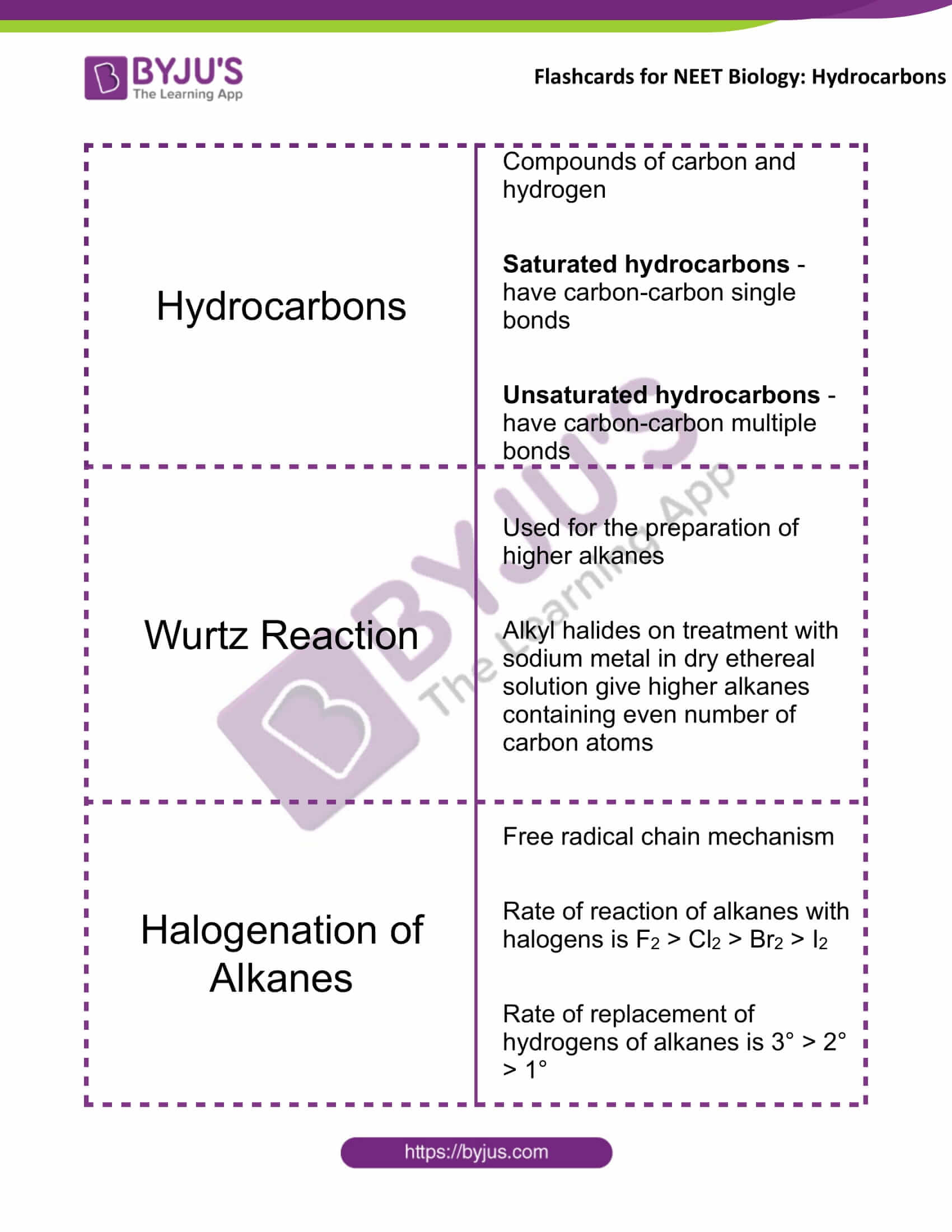
Hydrocarbons |
Compounds of carbon and hydrogen Saturated hydrocarbons – have carbon-carbon single bonds Unsaturated hydrocarbons – have carbon-carbon multiple bonds |
|
Wurtz Reaction |
Used for the preparation of higher alkanes Alkyl halides, on treatment with sodium metal in dry ethereal solution, give higher alkanes containing an even number of carbon atoms |
|
Halogenation of Alkanes |
Free radical chain mechanism Rate of reaction of alkanes with halogens is F2 > Cl2 > Br2 > I2 Rate of replacement of hydrogens of alkanes is 3° > 2° > 1° |
|
Controlled Oxidation of Alkanes |
Alkanes give a variety of oxidation products with suitable catalysts, e.g.
|
|
Markovnikov Rule |
Addition of hydrogen halides to unsymmetrical alkenes The negative part of the addendum (hydrogen halide, here) gets attached to the carbon atom having a lesser number of hydrogen atoms |
|
Peroxide or Kharash Effect |
Anti Markovnikov addition of HBr to unsymmetrical alkenes. It proceeds via free radical chain mechanism |
|
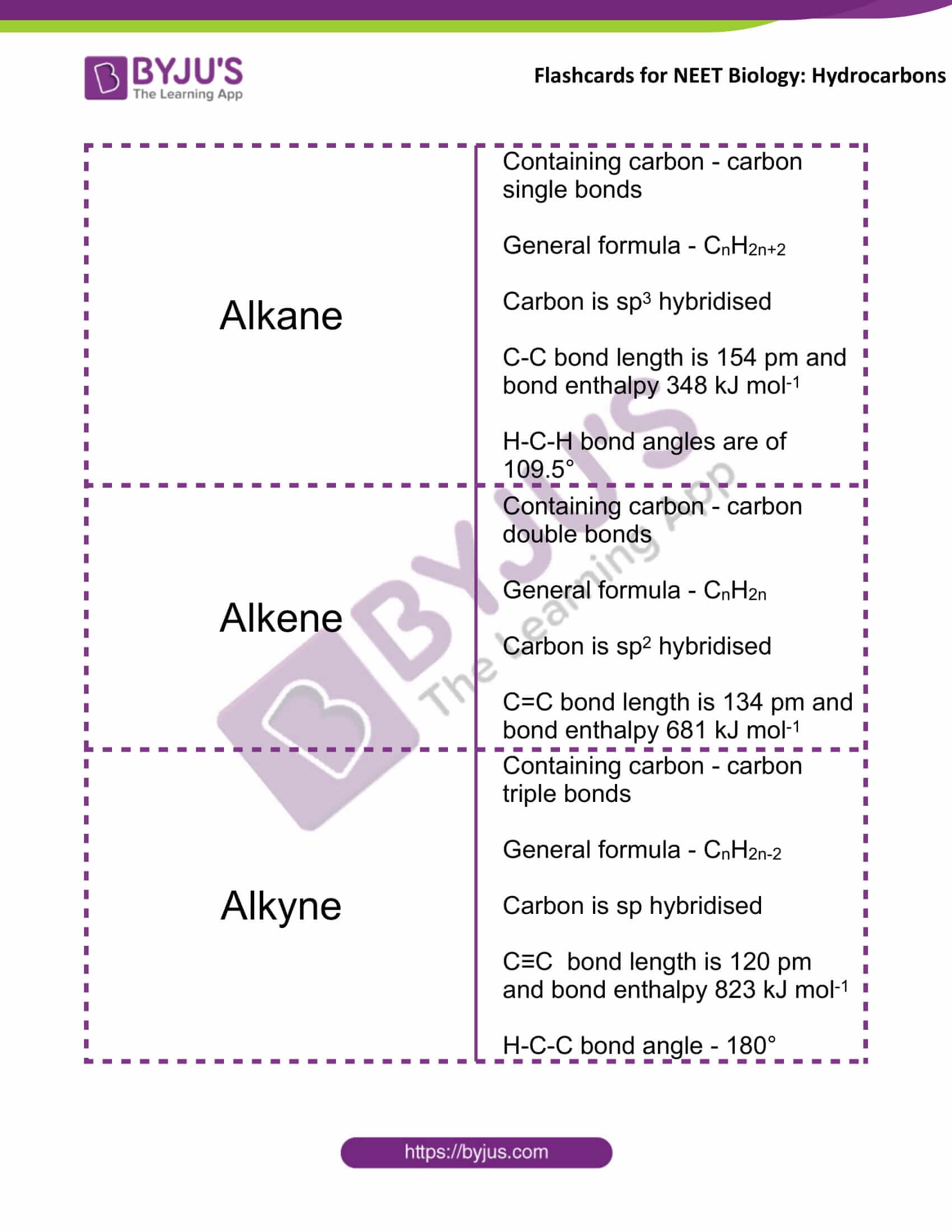
Alkane |
Containing carbon-carbon single bonds General formula – CnH2n+2 Carbon is sp3 hybridised C-C bond length is 154 pm and bond enthalpy 348 kJ mol-1 H-C-H bond angles are of 109.5° |
|
Alkene |
Containing carbon-carbon double bonds General formula – CnH2n Carbon is sp2 hybridised C=C bond length is 134 pm and bond enthalpy 681 kJ mol-1 |
|
Alkyne |
Containing carbon-carbon triple bonds General formula – CnH2n-2 Carbon is sp hybridised C≡C bond length is 120 pm and bond enthalpy 823 kJ mol-1 H-C-C bond angle – 180° |
|
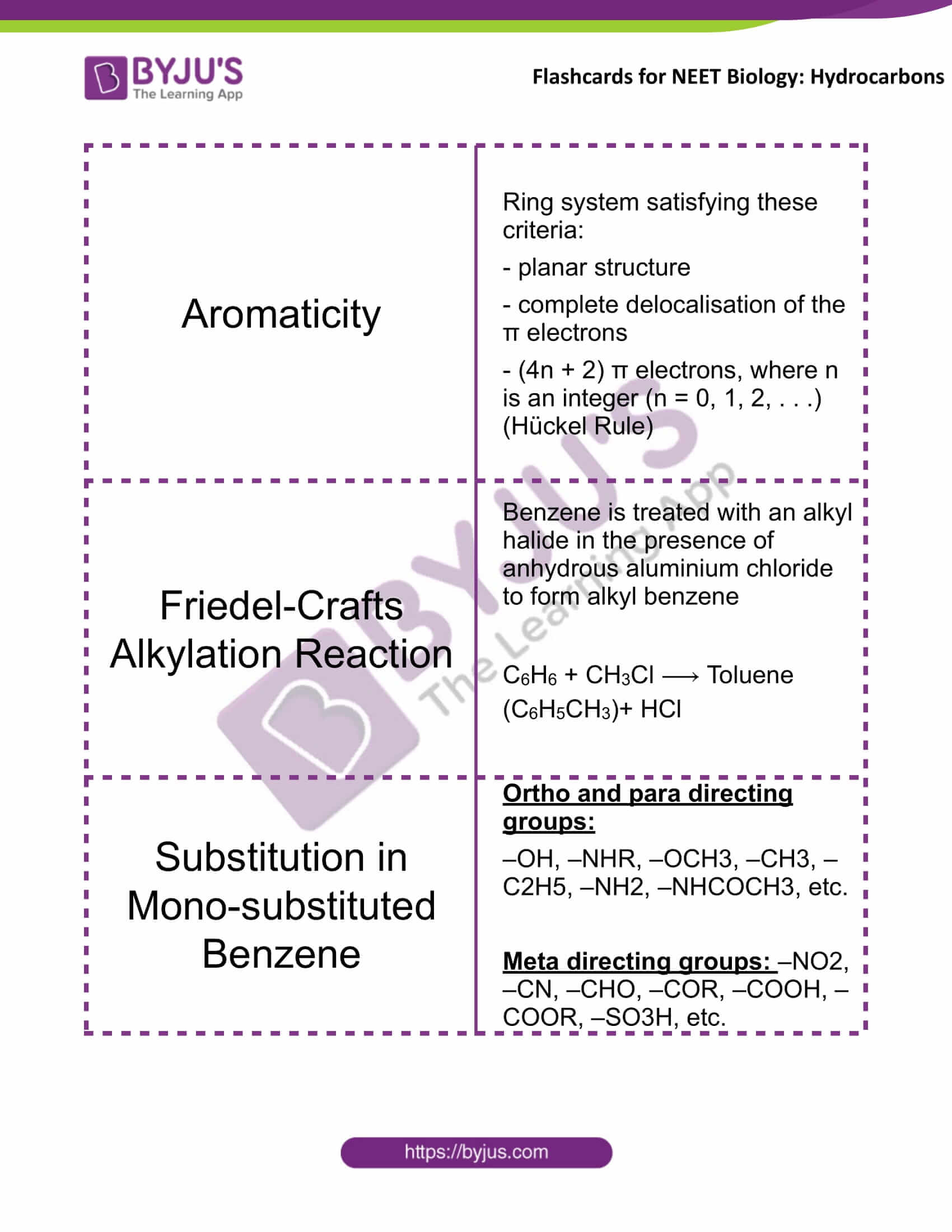
Aromaticity |
Ring system satisfying these criteria: – planar structure – complete delocalisation of the π electrons – (4n + 2) π electrons, where n is an integer (n = 0, 1, 2, . . .) (Hückel Rule) |
|
Friedel-Crafts Alkylation Reaction |
Benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride to form alkyl benzene C6H6 + CH3Cl ⟶ Toluene (C6H5CH3)+ HCl |
|
Substitution in Mono-substituted Benzene |
Ortho and para directing groups: –OH, –NHR, –OCH3, –CH3, –C2H5, –NH2, –NHCOCH3, etc. Meta directing groups: –NO2, –CN, –CHO, –COR, –COOH, –COOR, –SO3H, etc. |
Get access to the full set of flashcards for NEET Chemistry, only at BYJU’S.
|
Also check: |
Recommended Video:
Stability and Rearrangement of Carbocations Class 11 & 12 Chemistry | NEET 2022 Chemistry Exam Prep


Comments