Flashcards for NEET Chemistry are designed to boost your NEET preparation. Find below flashcards for the chapter “The s-Block Elements ”. These flashcards are prepared as per the NEET syllabus. These are helpful for aspirants of NEET and other exams during last-minute revision. It covers all the important points that are frequently asked in the exam. Check BYJU’S for the full set of Flashcards and Study material for NEET Chemistry.
Download PDF of NEET Chemistry Flashcards for the s-Block Elements
|
Name of the NEET Sub-section |
Topic |
Flashcards Helpful for |
|
Chemistry |
The s-Block Elements |
NEET Exams |
|
The s-Block Elements |
|
|
s-Block Group 1 Alkali Metals |
Outer electronic configuration – ns1 Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs) and Francium (Fr) They are called alkali metals because their hydroxides are strongly alkaline |
|
s-Block Group 2 Alkaline Earth Metals |
Outer electronic configuration – ns2 Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra) They are called alkaline earth metals (exception Be), because their oxides are found in the earth’s crust and their oxides and hydroxides are alkaline. |
|
Alkali Metals Reactivity |
Highly reactive due to large size and low ionisation enthalpy Reactivity increases down the group |
|
Chemical Properties of Alkali Metals |
They burn vigorously in oxygen forming oxides React with water to form hydroxide and dihydrogen They are strong reducing agents |
|
Anomalous Behaviour of Lithium |
It is due to the small size and high polarising power In the air, it forms mainly monoxide, Li2O and the nitride, Li3N Does not form ethynide Shows diagonal relationship to magnesium |
|
Important Compounds of Sodium |
Washing soda – Sodium carbonate, Na2CO3·10H2O Table salt – Sodium chloride, NaCl Caustic Soda – Sodium hydroxide, NaOH Baking Soda – Sodium Hydrogen carbonate, NaHCO3 |
|
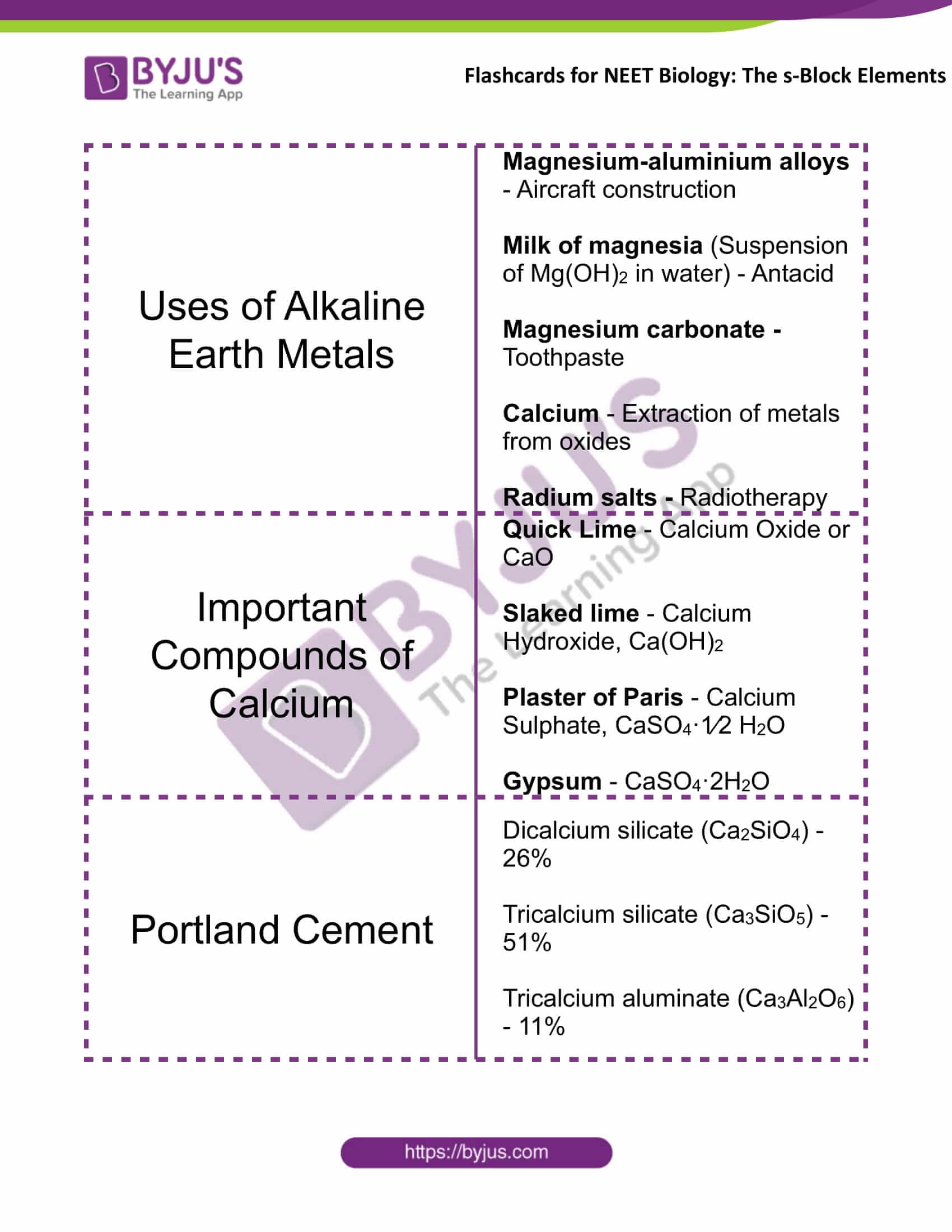
Uses of Alkaline Earth Metals |
Magnesium-aluminium alloys – Aircraft construction Milk of magnesia (Suspension of Mg(OH)2 in water) – Antacid Magnesium carbonate – Toothpaste Calcium – Extraction of metals from oxides Radium salts – Radiotherapy |
|
Important Compounds of Calcium |
Quick Lime – Calcium Oxide or CaO Slaked lime – Calcium Hydroxide, Ca(OH)2 Plaster of Paris – Calcium Sulphate, CaSO4·1⁄2 H2O Gypsum – CaSO4·2H2O |
|
Portland Cement |
Dicalcium silicate (Ca2SiO4) – 26% Tricalcium silicate (Ca3SiO5) – 51% Tricalcium aluminate (Ca3Al2O6) – 11% |
|
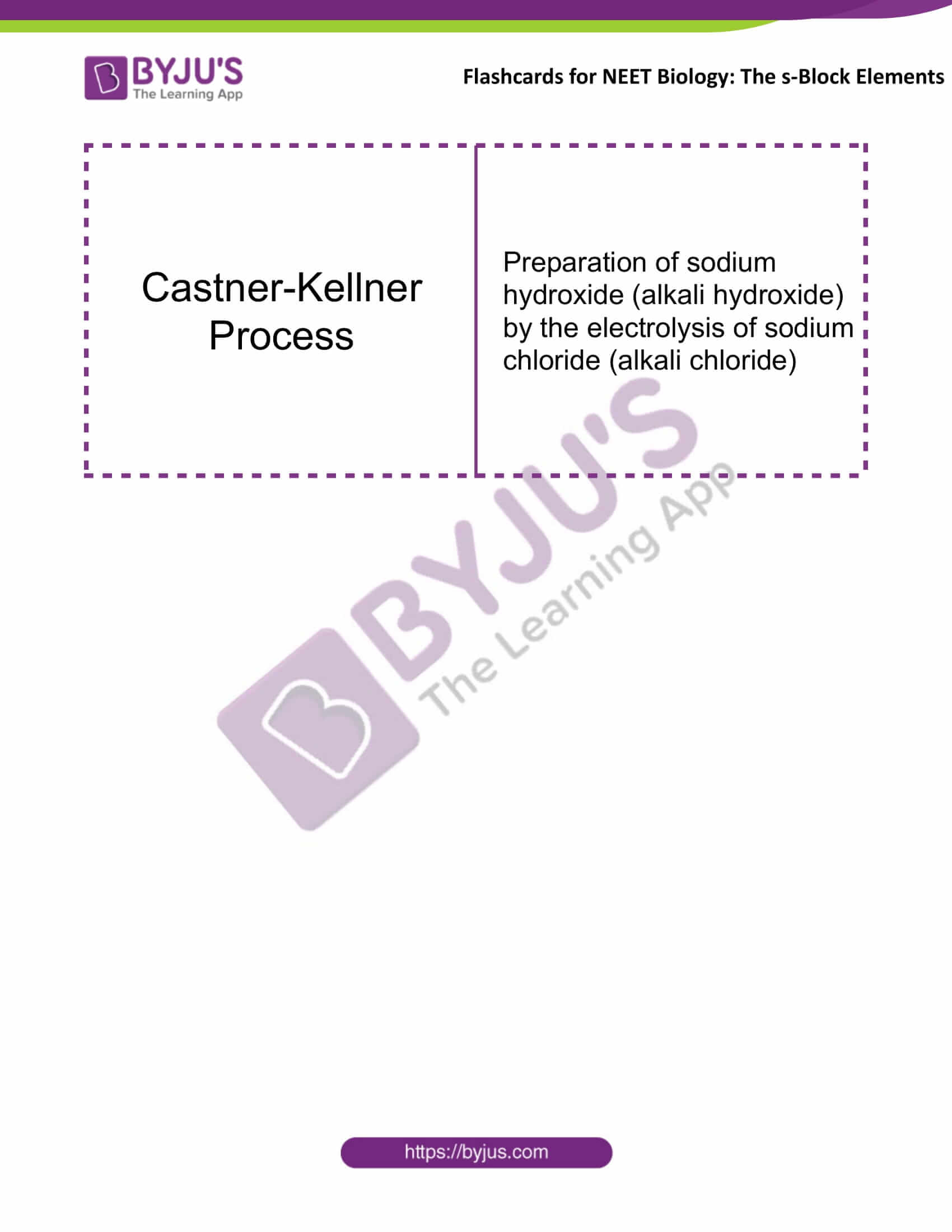
Castner-Kellner Process |
Preparation of sodium hydroxide (alkali hydroxide) by the electrolysis of sodium chloride (alkali chloride) |
Get access to the full set of flashcards for NEET Chemistry, only at BYJU’S.
Recommended Videos:


|
Also Check: |


Comments