Adenosine triphosphate or ATP is the energy currency of the cell. ATP hydrolysis releases the energy present in the high-energy terminal phosphate bonds, which is utilised to carry out various cellular reactions, such as muscle contraction, carbon fixation, etc. Various reactions are coupled with ATP hydrolysis.
ATP hydrolysis is an exergonic process. It produces ADP (Adenosine diphosphate), Pi (inorganic phosphate) and energy. ADP can undergo further hydrolysis in some cases to produce AMP (Adenosine monophosphate) and Pi.
ATP Structure and Hydrolysis Mechanism
ATP is ribonucleic acid. It is made up of a nitrogenous base, pentose sugar and phosphate. In ATP, the nitrogenous base is adenosine and the sugar is ribose, which is linked to phosphate.
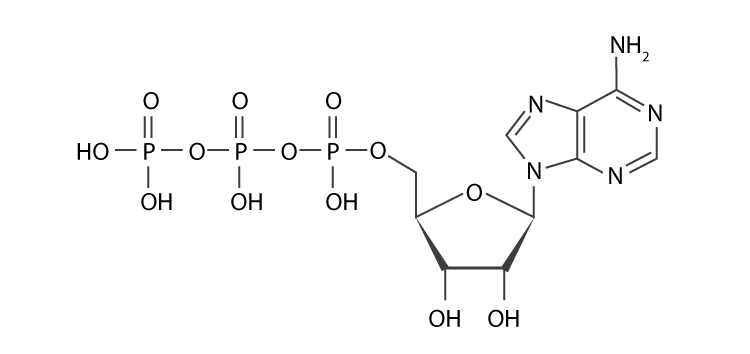
ATP Hydrolysis
The terminal phosphoanhydride bonds are known as high-energy bonds. They release a large amount of energy on hydrolysis to power the energy-requiring cellular processes.
The reaction of ATP hydrolysis is as follows:
ATP + H2O ⇋ ADP + Pi + Energy
This reaction is reversible. The reversible reaction requires energy to produce ATP. ADP and Pi regenerate ATP. Thus, energy gets stored in the form of ATP and when energy is required, ATP is hydrolysed. The reverse reaction of ATP hydrolysis or the reaction for ATP synthesis is as follows:
ADP + Pi + Energy ⇋ ATP + H2O
The enzyme ATP synthase catalyses the synthesis of ATP.
Energy Output in ATP Hydrolysis
The hydrolysis of 1M of ATP into ADP and inorganic phosphate, releases −7.3 kcal/mol of energy. The energy released in the living cell is almost double the value in standard conditions, it is equal to −14 kcal/mol.
ATP + H2O → ADP + Pi ΔG° = −7.3 kcal/mol (−30.5 kJ/mol)
The hydrolysis of ATP into AMP and pyrophosphate (PPi) releases −10.9 kcal/mol of energy.
ATP + H2O → AMP + PPi ΔG° = −10.9 kcal/mol (−45.6 kJ/mol)
Significance of ATP Hydrolysis
Energy is stored in the form of ATP in living organisms. Most ATP is produced during respiration. The energy released by the oxidation of carbohydrates and respiratory substrates is trapped and stored in the form of ATP, which can later be utilised as and when the requirement arises.
The energy released during ATP hydrolysis is utilised to power the cellular processes. There are many processes that require energy, such as muscle contraction, active transport, cell signalling, DNA RNA synthesis, etc.
There are many reactions that are coupled to ATP hydrolysis. These reactions are endergonic, i.e. they require energy. An example of reaction coupling is phosphorylation, e.g. glucose to glucose 6 phosphate catalysed by the enzyme hexokinase in the glycolysis process. The phosphate group released in ATP hydrolysis is used to phosphorylate glucose. Sodium-potassium pump is another example where ATP hydrolysis is coupled to the change of shape of transport proteins, leading to transport of ions across the membrane.
This was all about ATP Hydrolysis. Explore notes on other important concepts related to NEET, only at BYJU’S.
Further reading:
- What Type Of Biomolecule Is ATP?
- What Is the Net ATP Gain from One Glucose?
- How Is ATP Produced in Prokaryotic Cells?
- Does ATP Synthesis Require Oxygen?
- How Do Plants Produce ATP?
Recommended Video:
Biomolecules Class 11 Biology (Chapter 9) | Important Questions for NEET Zoology 2022 Exam
Comments