The term oxidation refers to any chemical change in which there is an increase in oxidation number while the term reduction refers to any change resulting in decrease in oxidation number. Oxidation of metals or alloys takes place when they are heated in a highly oxidizing atmosphere such as air or oxygen.
Potassium permanganate – KMnO4
This is the best oxidizing chemical to use for manganese control removal. As an extremely strong oxidant, it has the additional benefit of producing manganese dioxide during the oxidation reaction.
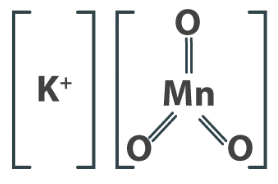
Potassium permanganate is a strong oxidant. During oxidation, potassium permanganate is reduced to insoluble manganese dioxide or to the manganous ion (Mn2+). The rates of oxidation reactions of MnO4– are greatly influenced by pH as shown below.
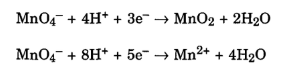
Reaction on C=C double bonds
Permanganate can react with a C=C double bond by hydroxylation. Instead of the chain cleavage, hydroxyl groups are added, resulting in a glycol formation.
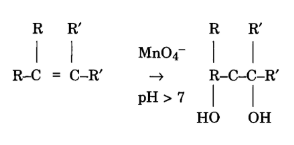
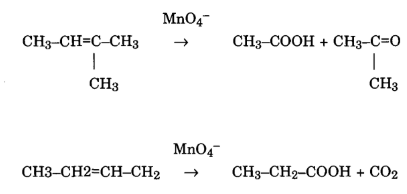
Reactions with Functional Groups
The reactivity of permanganate with alcohols depends on the location of the hydroxyl group in the molecule.
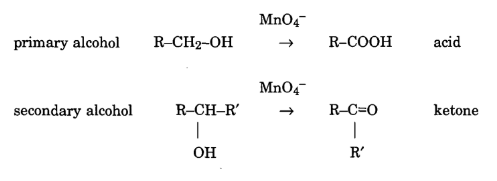
Permanganate can also oxidise aldehyde to acids.
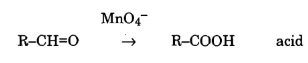
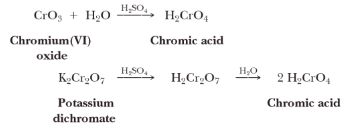
Potassium Dichromate – K2Cr2O7
A reagent commonly used in laboratories for the oxidation of primary alcohol to a carboxylic acid is chromic acid, H2CrO4. Chromic acid is prepared by dissolving either chromium (VI) oxide or potassium dichromate in aqueous sulfuric acid.
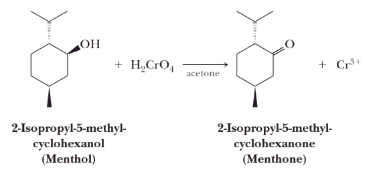
Secondary alcohols are oxidized to ketones.
Periodic Acid – HIO4
Periodic acid is known to oxidise substances other than carbohydrates to form reactive aldehydes. The amino alcohols of serine and threonine are oxidised by periodic acid but only when present at the end of the protein chain.
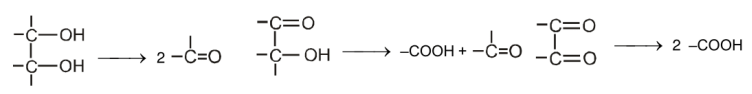
Baeyer’s Reagent
Baeyer’s reagent is a dilute alkaline KMnO4 solution, which is purple in colour. In this test the alkene is oxidised to a diol and alkyne is oxidised to carboxylic acids which use up the purple permanganate solution and produces manganese dioxide. This disappearance of purple colour is the indication of the presence of unsaturation or C-C double or triple bonds in molecules.
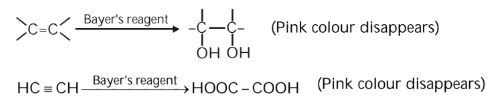
Baeyer-Villiger Oxidation
In Baeyer Villiger oxidation a carbonyl compound and an aromatic peracid react via insertion of the peroxo-O atom next to the C=O bond of the carbonyl compound.
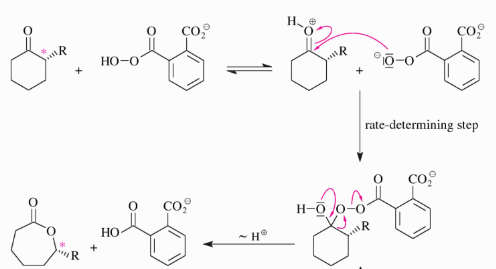
The Baeyer–Villiger Reaction is a commonly used oxidation reaction in organic synthesis is the conversion of carbonyl compounds into the corresponding esters or lactones.
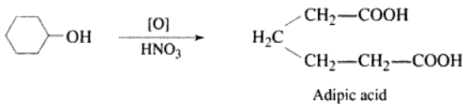
Nitric Acid – HNO3
Concentrated nitric acid takes part in the same reactions as dilute nitric acid and also acts as an oxidising agent. It is an even more powerful oxidising agent than concentrated sulphuric acid. The oxidising property of nitric acid is based on the fact that – when nitric acid undergoes decomposition, it yields – nascent oxygen.
Thus oxidation proceeds with loss of electrons while reduction is accompanied by a gain of electrons. The oxidising agent is by definition the substance containing the atom which shows a decrease in oxidation number, while a reducing agent is a substance containing the atom which shows an increase in oxidation number.
Most searched topics on NEET Chemistry:
- NEET Chemistry Syllabus
- How to Score 160 Plus in NEET Chemistry
- NEET Chemistry Weightage
- Chemistry Formulas for NEET
- NEET Chemistry MCQs
- NEET Chemistry Important Topics
Comments