Pyruvate is the conjugate base of pyruvic acid. It is a key intermediate in many biological processes.
It is produced at the end of the glycolysis process and is the connecting link of various biochemical processes such as gluconeogenesis, fermentation, cellular respiration, fatty acid synthesis, etc.
What is Pyruvic acid?
It is the simplest ɑ-keto acid that has carboxylic acid and ketone as its functional group. When pyruvic acid donates a proton, it forms a conjugate base called pyruvate. Pyruvate is seen as an intermediate in most metabolic pathways.
Pyruvic acid smells more like acetic acid. Also, pyruvic acid is colourless and is miscible with water.
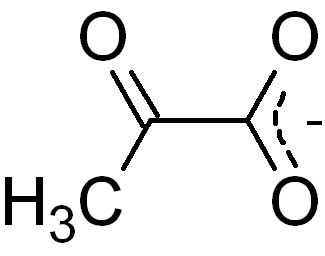
Pyruvic acid formula – CH3COCOOH
Pyruvate Formula
Pyruvate formula is CH3COCOO–.
Biochemical Reactions involving Pyruvate
Pyruvate is a key intermediate in various metabolic processes in living organisms.
It is an important topic from the NEET perspective. See below for important metabolic reactions involving pyruvate.
| Types of Reactions | Biochemical Process | Reactions Involving Pyruvate | Enzymes Involved |
| Pyruvate Synthesis | Glycolysis | Glycolysis is the first step in cellular respiration. Glucose is converted to pyruvate in a series of reactions.
Phosphoenolpyruvate or PEP is converted to pyruvate. |
PEP carboxylase |
| Pyruvate Carboxylation | Gluconeogenesis | Gluconeogenesis is the process of producing glucose from non-carbohydrate sources. The non-carbohydrate sources are first converted to pyruvate and then to glucose.
Pyruvate is carboxylated to form oxaloacetate. |
Pyruvate carboxylase |
| Pyruvate Oxidation or Decarboxylation | Synthesis of Acetyl-CoA | Acetyl-CoA formed from pyruvate carboxylation enters the Krebs cycle in aerobic respiration.
Acetyl-CoA thus formed is a key intermediate for fatty acid synthesis. |
Pyruvate dehydrogenase complex |
| Pyruvate Reduction | Anaerobic Respiration and Fermentation | Pyruvate is converted to lactate in animals anaerobically.
In alcoholic fermentation, Pyruvate is converted to acetaldehyde and eventually to ethanol. |
Lactate dehydrogenase
Pyruvate decarboxylase |
| Pyruvate Transamination | Formation of amino acid | Pyruvate gets converted to alanine by transamination.
Pyruvate and glutamate get converted into alanine and α-ketoglutarate, respectively. |
Alanine transaminase (ALT) |
This was a brief note on Pyruvate. Explore notes on Glycolysis and other important concepts related to NEET, only at BYJU’S.
Frequently Asked Questions
What is the main function of pyruvate?
Pyruvate is produced at the end of the glycolysis process and is a key intermediate in various metabolic pathways such as gluconeogenesis, fermentation, cellular respiration, fatty acid synthesis, etc. Pyruvate provides energy to the living cells through Kreb’s cycle. Pyruvate is first decarboxylated to acetyl CoA and then acetyl CoA enters the Krebs cycle.
How is pyruvate used in the body?
Pyruvate is used in various metabolic pathways. Some of the examples are:
- Energy production during aerobic respiration: Pyruvate produced in the glycolysis enters mitochondria and after oxidative decarboxylation, acetyl CoA is produced, which enters the Citric acid cycle for energy production.
- Fermentation: During anaerobic respiration, pyruvate is converted into alcohol and lactic acid.
- Formation of carbohydrates: Carbohydrate is produced by gluconeogenesis. In this process, glucose is produced from non-carbohydrate sources. The non-carbohydrate sources are first converted to pyruvate and then to glucose.
- Formation of amino acid: Pyruvate gets converted to alanine by transamination.
What is pyruvate the product of?
Glucose is converted into pyruvate by a series of reactions through glycolysis. Glycolysis is the first step of cellular respiration.
What is pyruvate also known as?
Pyruvate is the conjugate base of pyruvic acid. It is also known as the a-keto propanoic acid.
Important MCQs for NEET Biology:

Comments