The Cannizzaro reaction is a reaction of organic compounds having an aldehydic group (-CHO). It was propounded by Stanislao Cannizzaro in 1853 and hence was named after him. We have already studied aldol condensation, which is also an organic compound reaction having an aldehydic group which contains α-hydrogen in the presence of dilute alkali, giving β-hydroxy aldehyde as a condensation product. The product contains hydroxy (-OH) and aldehyde (-CHO) group, hence it is known as aldol condensation. The Cannizzaro reaction differs from the aldol with respect to the α-hydrogen.
Requirements for the Cannizzaro Reaction
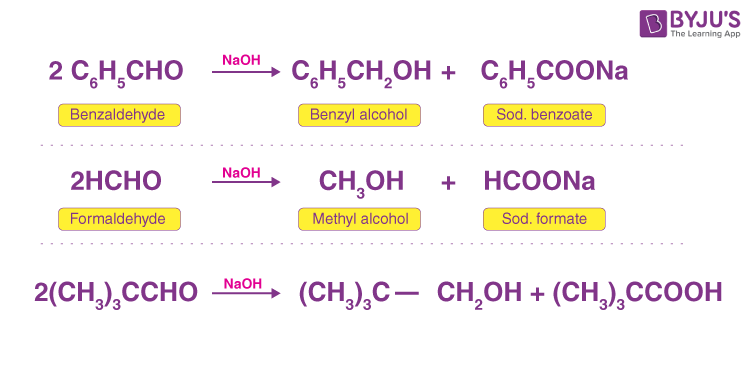
- Aromatic aldehydes do not contain α-hydrogen; for example, C6H5CHO (benzaldehyde).
- Aliphatic aldehydes do not contain α-hydrogen; for example, HCHO, (CH3)3CCHO.
- Presence of concentrated aqueous alkali (KOH, NaOH).
The following products are formed:
- Reactants undergo self-oxidation and reduction to give alcohol and the salt of the corresponding carboxylic acid.
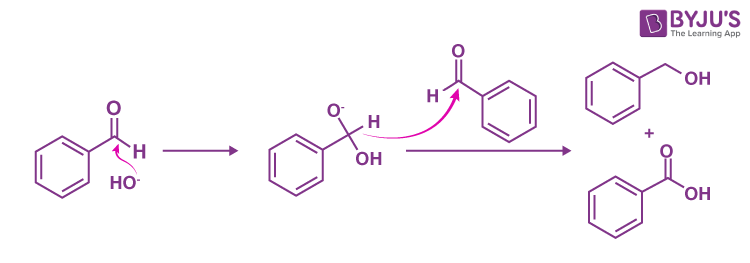
The reaction involved is as follows:
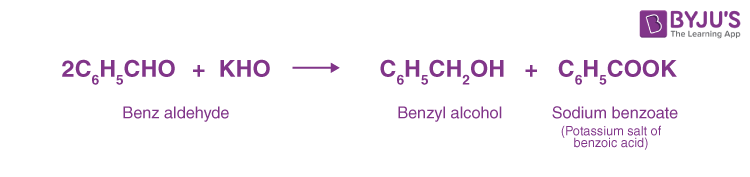
OR
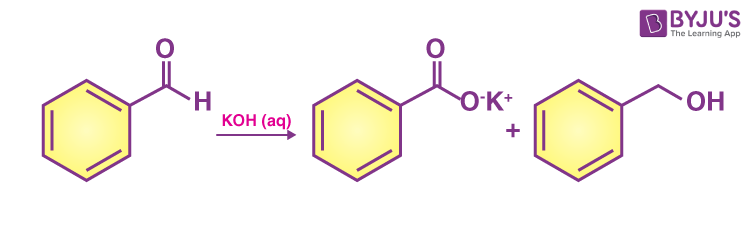
This disproportionation reaction (same reactant undergoing oxidation and reduction reaction) of aldehydes without α-hydrogen is known as the Cannizzarro reaction.
The Mechanism for the Reaction
Step 1:
- In this step, OH–(hydroxide ion), which is a nucleophile and attacks the carbonyl carbon of the aldehydic group to give a reducing anion (1).
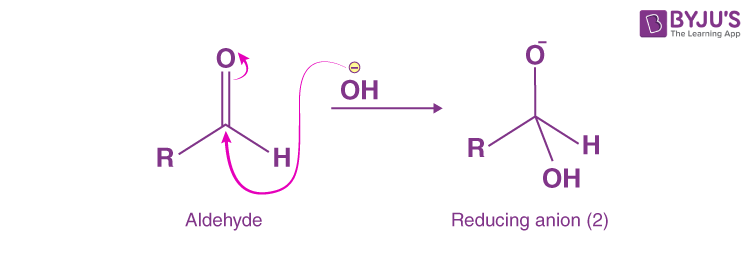
Step 2:
- The anion formed in the first reaction transfers a hydride ion to the carbonyl carbon of another molecule of the same aldehyde, as used in step 1, forming carboxylic acid and the alkoxide ion.
- The shift of proton from the acid to (2) gives the final product as alcohol.
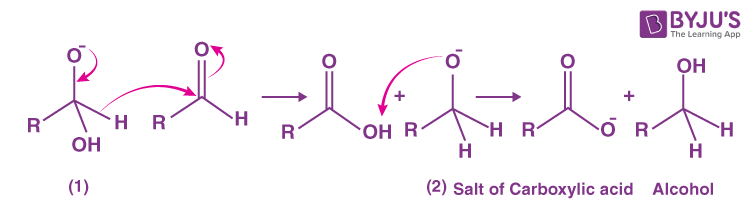
OR
Examples of Cannizzaro’s reaction are given below for better understanding.
Applications of Cannizzaro Reaction
- Formation of carboxylic acid and alcohol from aldehydes devoid of α-hydrogen.
Variations of This Reaction for Further Study
-
- Crossed Cannizzaro Reaction:
- This reaction basically includes a mixture of two aldehydes having no α-hydrogen to yield all the products which are possible from the reactants used.
- The reaction between benzaldehyde and sodium formate is given below to understand the reaction easily.
- Crossed Cannizzaro Reaction:
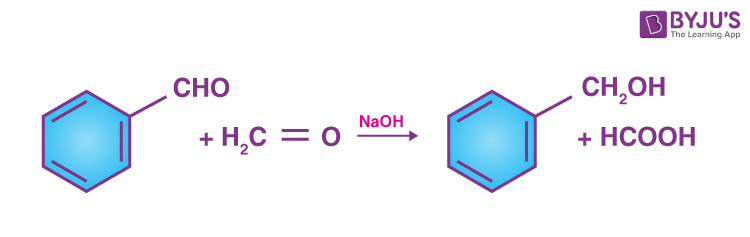
-
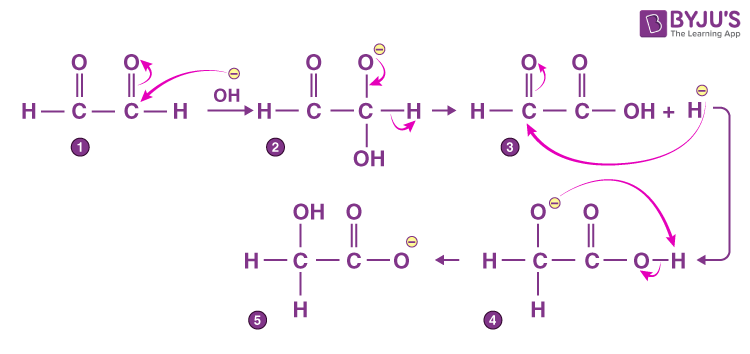
- Intramolecular Cannizzaro Reaction:
- This reaction is within the same compound in which two aldehydic groups are present, which are devoid of α-hydrogen.
- An example is glyoxal having two aldehydic groups, the reaction mechanism for the same is given below.
- Intramolecular Cannizzaro Reaction:
Question to Practise
Q1. Crossed Cannizzaro reaction is given by:
- Between two molecules of glyoxal
- Between acetone and acetaldehyde
- Between formaldehyde and benzaldehyde
- Between two molecules of formaldehyde

Comments