Here we are providing a solved KVPY-SA 2020 Chemistry question paper and this paper can prove to be a good resource for preparing productively for the upcoming examination. Solutions for each question are given in a detailed manner so that students will find it easier to understand each topic covered in the question paper.
Students can also find out what type of questions are asked in KVPY question papers, the exam pattern and more. They will also get a much clearer idea of the questions they can expect in the upcoming KVPY exam. Students can download this question paper and try solving the question without referring to the answers. This way, they can get enough practice and also develop better speed and precision to solve questions. View and download the KVPY-SA 2020 Chemistry question paper pdf from below.
Question 1:The acidity of

Follows the order
a. I > II > III > IV
b. IV >III > II > I
c. III > IV > I > II
d. III > II > IV > I
Answer: (c)
The presence of -M/-I groups increases the acidic nature since it stabilizes the negatively charged conjugate base by resonance or inductive effect. +M groups decrease the acidic nature since it destabilizes the negatively charged conjugate base by resonance.

So, the order of acidity is;
III > IV > I > II
Question 2: Among the following,

the compounds which can exhibit optical activity are:
a. only II, IV and V
b. only IV and V
c. only I, II and V
d. only I, II and IV
Answer: (a)
If the plane of symmetry and the centre of symmetry are absent, then the compound is optically active.
Compounds II, IV and V do not have a plane of symmetry as well as a centre of symmetry. Hence they are optically active.
I) Centre of symmetry is present.
III) Plane of symmetry is present
Question 3: A molecule that has 10, 20 and 30 carbon atoms is:
a. 2,3,4-trimethylpentane
b. chlorocyclohexane
c. 2,2-dimethylcyclohexane
d. methylcyclohexane
Answer: (d)

Methylcyclohexane:
Number of 10 carbon atoms = 1
Number of 20 carbon atoms = 5
Number of 30 carbon atoms = 1
Question 4: The organic compound which can be purified by steam distillation is:
a. acetone
b. aniline
c. glucose
d. ethanol
Answer: (b)
Aniline is insoluble in water and steam volatile, thus purified by steam distillation.
Question 5: Among the following, the most acidic compound is:

Answer: (b)

Aromatic compounds are more stable than non-aromatic compounds.
Question 6: A closed 10 L vessel contains 1 L water gas (1:1 CO:H2) and 9 L air (20% O2 by volume) at STP. The contents of the vessel are ignited. The number of moles of CO2 in the vessel is closest to:
a. 0.22
b. 0.022
c. 0.90
d. 3.60
Answer: (b)
1L water gas (1:1 CO : H2)
∴VCO = 0.5 L
VH2 = 0.5 L
Vair = 9L
= (20/100) ×Vair
= (20/100)×9 = 1.8L
The combustion reactions are:
CO + ½ O2 →CO2 H2 + ½ O2 → H2O
Here CO is limiting reagent
1 mol CO produce → 1 mole CO2
0.5 L CO produce → 0.5 L CO2
VCO2 produce = 0.5 L
Mole = 0.5/22.4 = 0.022
Question 7: A certain metal has a work function of Φ = 2 eV. It is irradiated first with 1W of 400 nm light and later with 1W of 800 nm light. Among the following, the correct statement is:
[Given: Planck constant (h)= 6.626 x 10–34 m2 kg s–1: Speed of light (c) = 3 × 108 m s–1]
a. Both colours of light give rise to the same number of photoelectrons.
b. 400 nm light gives rise to less energetic photoelectrons than 800 nm light.
c. 400 nm light leads to more photoelectrons.
d. 800 nm light leads to more photoelectrons.
Answer: (c)

According to photoelectric law
E = E0 + K.E.
E = hc/λ
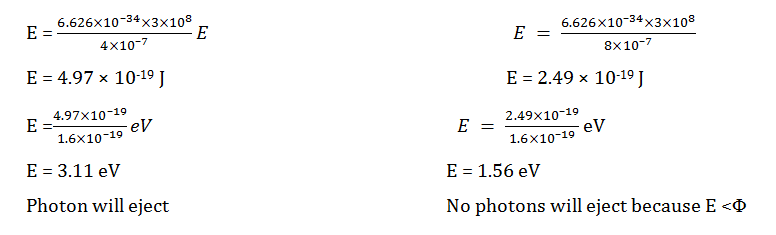
Question 8: Among the following, the correct statement about the chemical equilibrium is:
a. Equilibrium constant is independent of temperature.
b. Equilibrium constant tells us how fast the reaction reaches equilibrium.
c. At equilibrium, the forward and the backward reactions stop so that the concentrations of reactants and products are constant.
d. Equilibrium constant is independent of whether you start the reaction with reactants or products.
Answer: (d)
Equilibrium constant is dependent on temperature.
Equilibrium constant does not tell us about the rate of reaction.
At equilibrium, the forward and backward reactions do not stop but they have the same rate.
Question 9:Among the following, the plot that shows the correct marking of most probable velocity (VMP), average velocity (

Answer: (d)
Vmp =
Vrms= √(3RT/M)
By solving these we, get
Vmp<
Question 10: The correct set of quantum numbers for the unpaired electron of Cu atom is:
a. n = 3, l = 2,m = –2, s = + ½
b. n = 3, l = 2,m = +2, s = – ½
c. n = 4, l = 0, m = 0, s = + ½
d. n = 4, l = 1. m = +1, s = + ½
Answer: (c)
29Cu → 1s2 2s2 2p6 3s2 3p6 3d10 4s1
The set of quantum number for the unpaired electron of Cu atom is
n = 4, l = 0, m = 0, s = ±1/2
Question 11: Among the following, the most polar molecule is:
a. AlCl3
b. CCl4
c. SeCl6
d. AsCl3
Answer: (d)
AlCl3, CCl4, and SeCl6 have zero dipole moment so they are non-polar.
Question 12: The covalent characters of CaCl2, BaCl2, SrCl2 and MgCl2 follow the order:
a. CaCl2< BaCl2< SrCl2< MgCl
b. BaCl2< SrCl2< CaCl2< MgCl2
c. CaCl2< BaCl2< MgCl2< SrCl2
d. SrCl2< MgCl2< CaCl2< BaCl2
Answer: (b)
Polarization ∝ Charge/size ∝Covalent character
Order of polarizing power : Mg++> Ca++> Sr++> Ba++
Order of Covalent character : MgCl2> CaCl2> SrCl2> BaCl2
Question 13: Among the following, the correct statement is:
a. 100. has four significant figures
b. 1.00 × 102 has four significant figures
c. 2.005 has four significant figures
d. 0.0025 has four significant figures
Answer: (c)
A) 100 → 3 significant figure
B) 1.00 × 102 → 3 significant figure
C) 2.005 → 4 significant figure
D) 0.0025 → 2 significant figure
Question 14: A thermodynamic cycle in the pressure (P) – volume (V) plane is given below:

AB and CD are isothermal processes while BC and DA are adiabatic processes. The same cycle in the temperature (T) – entropy (S) plane is:

Answer: (a)
AB→ Isothermal expansion (i.e. TA = TB)
On moving A to B, volume increases and P decreases so, entropy will increase.
BC→ Adiabatic expansion, so cooling occurs.
So TB> TC
For the adiabatic process, ΔS = 0, which means entropy remains the same.
CD→ Isothermal compression (i.e. TC = TD)
On moving C to D, volume decreases and P increases so, entropy will decrease.
DA→ Adiabatic compression, so heating occurs.
So TA> TD
For the adiabatic process, ΔS = 0 means entropy remains the same.
Question 15: The first ionization potential (IP) of the elements Na, Mg, Si, P, Cl and Ar are 5.14, 7.65, 8.15, 10.49, 12.97 and 15.76 eV, respectively. The IP (in eV) of K is closest to:
a. 13.3
b. 18.2
c. 4.3
d. 6.4
Answer: (c)
On moving down in a group, ionization potential generally decreases. So, the ionization potential of K should be less than that of Na.
Question 16: A hydrocarbon X with molecular formula C4H6 decolorizes bromine water and forms a white precipitate in ethanolic AgNO3 solution. Treatment of X with HgCl2 in aqueous H2SO4 produces a compound, which gives a yellow precipitate when treated with I2 and NaOH. The structure of X is:

Answer: (d)
Terminal Alkyne gives Tollen’s Reagent Test and the compound has a ketomethyl group.

Question 17: 0.102 g of an organic compound X was oxidized with fuming nitric acid. The resulting solution, after reaction with an excess of aqueous BaCl2, produced 0.233 g of BaSO4 as a precipitate. Compound X is likely to be:
[Given: Atomic wt. of Ba = 137]

Answer: (d)
% of S in compound = (32×0.233)×100/(233×0.102) = 31.37%

Question 18: The specific heat of a certain substance is 0.86 J g–1 K–1. Assuming ideal solution behaviour, the energy required (in J) to heat 10 g of 1 molal of its aqueous solution from 300 K to 310 K is closest to:
[Given: molar mass of the substance = 58 g mol–1; specific heat of water = 4.2 J g–1K–1]
a. 401.7
b. 424.7
c. 420.0
d. 86.0
Answer: (a)
Specific heat capacity substance = 0.86 Jg-1 K–1
1 molal aqueous solution
⇒ 1058 g water has 1 mole (or 58 g) substance.
⇒ 10 g solution will have 0.54 g substance.
Weight of substance = 0.54 g
Weight of water = 10 – 0.54 = 9.46 g
According to law of calorimetry
qtotal = qsubstance + qwater
= (mSΔT)substance + (mSΔT)water
qtotal = [0.54 × 0.86 × (310 – 300)] + [9.46 × 4.2 × (310 – 300)]
qtotal = 4.64 + 397.32
qtotal = 401.96 ≈ 401.7 J
Choose the closest option
Question 19: Strength of a H2O2 solution is labelled as 1.79 N. Its strength can also be expressed as closest to:
a. 20 volume
b. 5 volume
c. 10 volume
d. 15 volume
Answer: (c)
N = (volume strength)/5.6
Volume strength = 1.79 × 5.6
Volume strength = 10.024
Question 20: The isotherms of gas are shown below:

Among the following.
(i) At T1, the gas cannot be liquified
(ii) At point B, the liquid starts to appear at T2
(iii) TC is the highest temperature at which the gas can be liquified
(iv) At point A, a small increase in pressure condenses the whole system to a liquid
the correct statements are:
a. only (i) and (ii)
b. only (i), (iii) and (iv)
c. only (ii), (iii) and (iv)
d. (i), (ii), (iii) and (iv)
Answer: (d)

Above critical temperature (TC), gas cannot liquefy even applying high pressure.
At point B, the liquid starts to appear at T2. While at point A, a small increase in pressure condenses the whole system to a liquid.
Comments