The electromagnetic spectrum, in simple terms, is defined as the range of all types of electromagnetic radiation. We shall learn about the concept in detail and understand all its underlying aspects in this lesson.
Download Complete Chapter Notes of Electromagnetic Waves
Download Now
| Table of Contents |
What Is the Electromagnetic Spectrum?
The electromagnetic spectrum is a range of frequencies, wavelengths and photon energies covering frequencies from below 1 hertz to above 1025 Hz, corresponding to wavelengths which are a few kilometres to a fraction of the size of an atomic nucleus in the spectrum of electromagnetic waves. Generally, in a vacuum, electromagnetic waves tend to travel at speeds which is similar to that of light. However, they do so at a wide range of wavelengths, frequencies and photon energies.
The electromagnetic spectrum consists of a span of all electromagnetic radiation which further contains many subranges, which are commonly referred to as portions. These can be further classified as infrared radiation, visible light or ultraviolet radiation.
Electromagnetic Waves in the Electromagnetic Spectrum
The entire range (electromagnetic spectrum) is given by radio waves, microwaves, infrared radiation, visible light, ultra-violet radiation, X-rays, gamma rays and cosmic rays in the increasing order of frequency and decreasing order of wavelength. The type of radiation and their frequency and wavelength ranges are as follows:
| Type of Radiation | Frequency Range (Hz) | Wavelength Range |
| Gamma-rays | 1020 – 1024 | < 10-12 m |
| X-rays | 1017 – 1020 | 1 nm – 1 pm |
| Ultraviolet | 1015 – 1017 | 400 nm – 1 nm |
| Visible | 4 x 1014 – 7.5 x 1014 | 750 nm – 400 nm |
| Near-infrared | 1 x 1014 – 4 x1014 | 2.5 μm – 750 nm |
| Infrared | 1013 – 1014 | 25 μm – 2.5 μm |
| Microwaves | 3 x 1011 – 1013 | 1 mm – 25 μm |
| Radio waves | < 3 x 1011 | > 1 mm |
The electromagnetic spectrum can be depicted as follows:
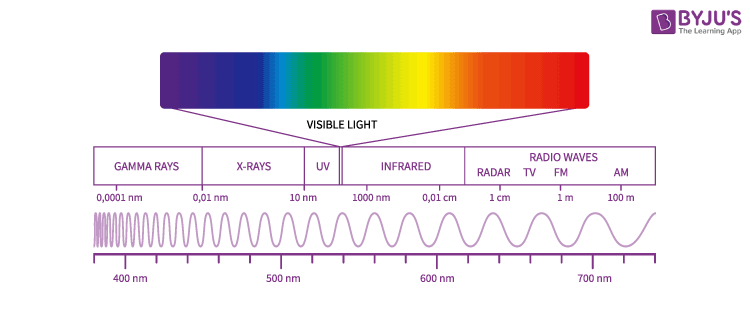
Let us look into the uses of electromagnetic waves in our daily life.
Radio: A radio basically captures radio waves that are transmitted by radio stations. Radio waves can also be emitted by gases and stars in space. Radio waves are mainly used for TV/mobile communication.
Microwave: This type of radiation is found in microwaves and helps in cooking at home/office. It is also used by astronomers to determine and understand the structure of nearby galaxies and stars.
Infrared: It is used widely in night vision goggles. These devices can read and capture the infrared light emitted by our skin and objects with heat. In space, infrared light helps to map interstellar dust.
X-ray: X-rays can be used in many instances. For example, a doctor can use an X-ray machine to take an image of our bones or teeth. Airport security personnel use it to see through and check bags. X-rays are also given out by hot gases in the universe.
Gamma-ray: It has a wide application in the medical field. Gamma-ray imaging is used to see inside our bodies. Interestingly, the universe is the biggest gamma-ray generator of all.
Ultraviolet: The Sun is the main source of ultraviolet radiation. It causes skin tanning and burns. Hot materials that are in space also emit UV radiation.
Visible: Visible light can be detected by our eyes. Light bulbs, stars, etc., emit visible light.
Spectroscopy: Spectroscopy is used to study the way different electromagnetic waves interact with matter.
We can learn about a substance by analysing the EM spectrum given by it. When light scatters or passes through matter, it tends to interact with molecules and atoms. Since atoms and molecules have resonance frequencies, they directly interact with those light waves having the exact frequencies. When collisions occur in an excited state, the atoms and molecules emit light with a certain set of characteristic frequencies. This further results in a line spectrum. Here, only light with detached wavelengths is produced. The spectrum is also not continuous, but it consists of a set of emission lines.
In cases where light with continuous wavelengths passes through a low-density material, the atoms and molecules of the material will absorb light waves with the same set of characteristic frequencies. This results in the production of the absorption spectrum, which is a nearly continuous spectrum with missing lines.
Significance of the Electromagnetic Spectrum
The electromagnetic waves in these different bands have different characteristics depending upon how they are produced, how they interact with matter and their practical applications. Maxwell’s equations predicted the existence of an infinite number of frequencies of electromagnetic waves, all travelling with the speed of light. This is the first indication of the existence of the entire electromagnetic spectrum.
Nonetheless, the main significance of the electromagnetic spectrum is that it can be used to classify electromagnetic waves and arrange them according to their different frequencies or wavelengths.
Practical Applications of Electromagnetic Waves
● The radio waves and microwaves discovered by Hertz paved the way for wireless television, radio and mobile communication.
● The visible light portion of the electromagnetic spectrum is the reason for all visual aids in daily life. This is the portion of the electromagnetic spectrum that helps us to see all objects, including colours.
● The X-rays discovered by Roentgen proved to be useful in medicine for detecting many ailments or deformities in bones.
● The high ultraviolet radiation has energies to ionise the atoms causing chemical reactions.
● The gamma rays discovered by Paul Villard are useful for ionisation purposes and nuclear medicine.
Formulas for the Electromagnetic Radiation
The frequency(f), speed(c), energy(E) and wavelength(λ) of electromagnetic waves are related as
![]()
Where,
● c = 299792458 m/s is the speed of light in a vacuum
● h = 6.62607015×10−34 J·s = 4.13566733(10)×10−15 eV·s is Planck’s constant.
Solved Questions
Question 1: What are the frequency and wavelength of an EM wave of energy 6.626 x 10-19 J?
Answer: Frequency(f) = E/h = 1015 Hz. Wavelength(λ) = c/f = 3 x 108 / 1015 = 3 x 107 metres
Question 2: What are the uses of X-rays?
Answer: X-rays can be used to detect medical ailments and bone deformities in medical diagnosis. They are useful for ionisation purposes also.
Question 3: Can we use X-rays and gamma rays for broadcasting radio/TV/mobile signals?
Answer: No, X-rays and gamma rays are short-range. Moreover, they are harmful and have penetrating power on the matter with which they interact and can damage the tissues of living bodies.
Question 4: Which among the following is not a property of electromagnetic waves?
1) Momentum
2) Energy
3) Pressure
4) Heat energy
Answer:
1) EM waves can impart momentum (and angular momentum) to the material with which it interacts.
2) Electromagnetic waves carry energy. EM waves are the only waves able to carry energy across a vacuum.
3) EM waves also exert pressure, which is shown by a radiometer. One side of the panels is black, the other side is white, and the panels spin due to the pressure differences under the light.
4) EM waves do not carry heat energy, but any EM radiation can heat an object when it is absorbed.
Question 5: A ray from the sun, passing through your kitchen window, hits a prism that casts a rainbow on the windowsill. Supposedly, there is a hand-held radiometer on the table. Now, you place the instrument on a specific colour of the rainbow with your eyes closed. When you open your eyes, you see that the radiometer measured the energy from that colour at 4.0 x 10-19 joules. If we take Planck’s constant of 6.6256 x 10-34 joules/sec, what possible colour did the instrument measure? How can we determine this?
Answer:
We should use the equation involving energy change, Planck’s constant, and frequency. Next, we need to figure out what we are solving for. In this problem, they ask for the possible colour that you measured. If we relate the energy with Planck’s constant, we can solve for frequency. We are given the energy, 4.0 x 10-19 joules, as well as Planck’s constant, 6.6256 x 10-34 joules/sec. Also, we are given the frequencies emitted by the visible spectrum, from red to violet. This problem is easy to solve now. If we solve for the frequency, we can then relate it to the energy emitted, measured in either sec-1.
Let’s solve it.
– What is the best equation to use? E = h* ƒ
– Solve for the intended variable. E/ h = ƒ
– Energy measured = 4.0 x 10-19 joules
Planck’s constant = 6.6256 x 10-34 joules/sec
Visible spectrum frequency
(4.0 x 10-19 joules) / 6.6256 x 10-34 joules/sec = ƒ
– Joules cancel out with joules, and one is left with sec-1, a frequency.
Answer = 6.03 x 1014 sec-1
This falls within the given visible spectrum frequencies. Being a high frequency on the visible spectrum, close to the frequencies emitted by blue colour, one could assume that you measured a colour close to blue, though cyan and green cannot be out of question as the given frequencies are a bit vague. It is closest to the green colour. Hence, we measured the green colour.
Rutherford’s Model and the Electromagnetic Spectrum

Frequently Asked Questions on Electromagnetic Spectrum
Which electromagnetic spectrum has the highest wavelength?
An electromagnetic spectrum is a collection of a range of waves in sequential order. Radio waves have the highest frequency, and gamma rays have the shortest wavelength.
Which part of the electromagnetic spectrum carries the least energy?
Radio waves have the lowest frequency and, hence, have the least energy.
E = hf
E = Energy
f = Frequency
h = Planck’s constant
Where in the electromagnetic spectrum is visible light?
Visible light falls in the range of the electromagnetic spectrum between infrared rays and ultraviolet rays. It has frequencies of about 4 × 1014 to 8 × 1014 hertz (Hz) and a wavelength of about 740 nanometers ( 2.9 × 10−5 inches) to 380 nanometers (1.5 × 10−5 inches).
Which part of the electromagnetic spectrum has the highest frequency?
Gamma rays have the highest frequency and the least wavelength.

Comments