Reduction is a chemical process that includes the gaining of electrons. For instance, rusting of iron, burning of combustible substances and fading of colour from clothes are some examples of reduction that can be witnessed in our daily life.
The net charge of an atom is reduced when very charged atoms are added to an atom. That is when a fluorine atom comprises an equal number of negatively charged electrons and positively charged protons, then the total charge will be equal to 0. When a fluorine atom gains an electron, it poses a new charge -1.
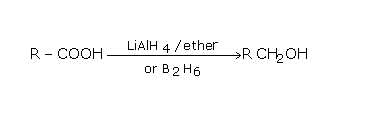
The above reaction illustrates the reduction reaction. Here, when the carboxylic acid is treated with aluminium hydride, they are reduced to the primary alcohol. The carboxylic acid will not be reduced by sodium borohydride.
Why Does Reduction Take Place?
Atoms will be fighting hard to maintain a balance between negatively charged electrons and positively charged protons. When these atoms lack electrons, they eventually grab electrons from a nearby source to attain electrical satisfaction. The certain electronic configuration is favourable, while certain are not. In order to attain a state of ideal electron configuration, atoms let go of the need to be neutrally charged.
A favourable electron configuration state is achieved when an atom has achieved a full octet. Some of the elements are near to achieving favourable electronic configuration resulting in a reduction of elements. The reduction process can be attained very easily for these elements, and they include bromine, oxygen, fluorine and chlorine.
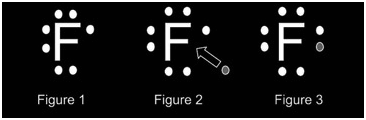
In the above diagram, figure one represents the element fluorine which possesses 7 electrons. At this stage, the element is neutrally charged. Figure two illustrates the gaining of the electron by fluorine. At this stage, the element is reduced. The figure represents a fluorine atom that has been reduced and comprises the full octet.

Comments