What Are Alkyl Halides?
Alkyl halides, also called haloalkanes or halogenoalkanes, are chemical compounds that are often derived from alkanes that contain one or more halogens. We can also say that alkyl halides are a subset of the general class of halocarbons.
Download Complete Chapter Notes of Haloalkanes and Haloarenes
Download Now
Alkyl halides or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms (Fluorine, chlorine, bromine or iodine). They can also be manufactured from any organic precursors such as alkanes, alkenes, alcohols and carboxylic acids. Generally, alkyl halides contain hydrogen atoms attached to the sp3 hybridized carbon atom of alkyl groups.
Some examples of alkyl halide include,
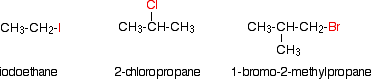
Classification of Alkyl Halide
Alkyl Halide can be classified on the basis of various aspects, and they are as follows.
Number of Halogen Atoms
Here, the classification mainly depends on whether they contain one, two, or more halogen atoms in their structure. Under this category, we have,
1. Mono Haloalkane
Example: CH3-CH2-X [Where X can be Cl, F, Br or I]
2. Dihaloalkane
Example: X-CH2-CH2-X [Where X can be Cl, F, Br or I]
3. Trihaloalkane
Example: X-CH2-CHX-CH2-X [Where X can be Cl, F, Br or I]
The Position of Halogen Atom Along the Chain of Carbon Atom
The classification depends on how the halogen atom is positioned on the chain of carbon atoms.
- Primary alkyl halide
- Secondary alkyl halide
- Tertiary alkyl halide
Primary Alkyl Halide
In this type of haloalkanes, the carbon which is bonded to the halogen family will be attached to only one other alkyl group. It doesn’t matter how much a bulky group is attached to it.
Some examples of primary haloalkanes are,
![]()
Secondary Alkyl Halide
In this type of haloalkanes, the carbon atom, which is bonded with the halogen atom, is joined directly to the other two alkyl groups, which can be the same or different. Some examples are,
![]()
Tertiary Alkyl Halide
In this type of haloalkanes, the carbon atom which carries the halogen atom is directly bonded to three alkyl groups. This alkyl group may be a combination of the same or different. Some examples are,
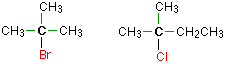
Alkyl Halide Properties
Alkyl halides are colourless when they exist in pure form. But, bromides and iodides develop colour when exposed to light. Many volatile halogen compounds have a sweet smell.
Boiling and Melting Points
- Methyl chloride, methyl bromide, ethyl chloride and some chlorofluoromethanes are in the form of gas at room temperature.
- Higher members are liquids or solids.
- As we know, molecules of organic halogen compounds are polar in nature.
- Due to greater polarity and greater molar mass as compared to the parent hydrocarbon, the intermolecular force of attraction is stronger in halogen derivatives.
- So, the boiling points of chlorides, bromides and iodides are considerably higher than that of the hydrocarbon with the same molecular mass.
- The attraction gets stronger as the size and number of electrons increase.
- The boiling points of alkyl halides will decrease in the order RI > RBr > RCl > RF.
Density
- Bromo-derivatives, iodo derivatives and polychloro derivatives of hydrocarbons are heavier than water.
- The density increases with an increase in the number of carbon atoms, halogen atoms and atomic mass of halogen atoms.
Solubility
- The haloalkanes are less soluble in water.
- To dissolve haloalkanes in water, energy is required to overcome the attraction between the haloalkane molecule and break the hydrogen bonds between the water molecules for haloalkanes.
- Very less amount of energy is released when new attractions between the haloalkanes and the water molecules are formed, which is not as strong as the original hydrogen bonds in water.
- So, the solubility of haloalkanes in water is less.
- But, the haloalkanes will dissolve in the organic solvent more than in the water. Because of this, the complex interaction between the haloalkanes and the creative molecules has the same potential as those broken by the unique and molecular haloalkanes.
Chemical Reactions
The chemical reaction of haloalkanes can be divided into three categories:
- Nucleophilic substitution reaction
- Elimination reaction
- Reaction with metals
Nucleophilic Substitution Reaction
In this type of reaction, a nucleophile reacts with haloalkane, which has a partial positive charge on the carbon atom that is bonded to halogen. A substitution reaction takes place, and the halogen atom called leaving group leaves as the halide ion. Since the substitution reaction is initiated by a nucleophile, it is called a nucleophilic substitution reaction.
Example:
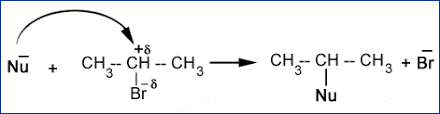
It is one of the most useful classes of organic reactions of an alkyl halide in which halogen is bonded to sp3 hybridized carbon.
Elimination Reaction
When a haloalkane having a hydrogen atom is heated with an alcoholic solution of potassium hydroxide, it will lead to the elimination of a hydrogen atom from the β-carbon atom and a halogen atom from the α-carbon atom. As a result, an alkene is formed as one of the products. Since the β-hydrogen atom is involved in elimination, it is often called a β-elimination reaction.
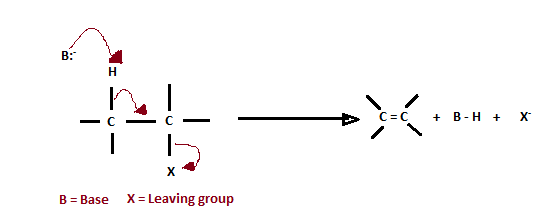
If there is any possibility of the formation of more than one alkene due to the presence of more than one β – hydrogen atom, usually one alkene is formed as the main product.
Reaction with Metals
Most organic chlorides, bromides and iodides react with certain metals to give compounds containing carbon-metal bonds. Such compounds are known as organometallic compounds. The product is formed by the reaction of haloalkanes with magnesium metal in dry ether.
On the other hand, Grignard reagents tend to react actively and can react with any source of protons leading to the formation of hydrocarbons. It is, therefore, essential to avoid the Grignard reagent. Otherwise, this will be considered as one of the modifications to hydrocarbons.
Synthesis of Alkyl Halides
Also Read: Preparation of Alkyl Halides
Uses of Alkyl Halide
- Many organic compounds containing halogens occur in nature, and some of them are clinically useful.
- These classes of compounds find good applications in industry as well as in day-to-day life.
- They are used as solvents for relatively non-polar compounds and as starting materials for the synthesis of a wide range of organic compounds.
- A chlorine-containing antibiotic, chloramphenicol, produced by soil microorganisms, is very effective for the treatment of typhoid fever.
- Some fully fluorinated compounds are considered potential blood substitutes in surgery.
- They are used as synthon equivalents in organic synthesis.
- They were previously used as refrigerants and propellants.
- They are also used in fire extinguishers.


Comments