What Is a Salt Bridge?
A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution.
Download Complete Chapter Notes of Electrochemistry
Download Now
The salt bridge usually consists of a strong electrolyte which is further made up of ions. For example, AgNO3, KCl, etc. Salt bridges are generally used in a galvanic cell such as a voltaic cell or a Daniel cell.
Salt Bridge Function
The main function of a salt bridge is to help maintain electrical neutrality within the internal circuit. It also helps in preventing the cell from taking its reaction to equilibrium. If salt bridges are absent or if they are not used, then the reaction will likely continue, and the solution in one-half electrodes will gather a negative charge. Similarly, in the other half, electrodes will accumulate a positive charge. This will further result in the stoppage of the reaction, and no electricity will be produced.
Therefore, a salt bridge basically helps in preventing the accumulation of positive and negative charges around the respective electrodes and further allows a smooth reaction to take place. It also helps in the continual flow of electrons. However, the purpose of a salt bridge is not to move electrons from the electrolyte; rather, it’s to maintain charge balance because the electrons are moving from one-half cell to the other.
- A salt bridge prevents the diffusion or mechanical flow of solution from one-half cell to another.
- It prevents or minimises the liquid-liquid junction potential. (Potential arises between two solutions when they are in contact with each other).
- A salt bridge acts as an electrical contact between two half-cells.
Types of Salt Bridges
There are mainly two types of salt bridges used in electrochemical cells.
- Glass Tube Bridge
- Filter Paper Bridge
Glass Tube Bridge
They are generally U-shaped tubes filled with electrolytes. Sodium Chloride (NaCl), Potassium Chloride (KCl), and Potassium Nitrate (KNO3) are generally used electrolytes. The electrolyte needs to be relatively unreactive with other chemicals in the cell and have cations and anions with similar migratory speeds (comparable ion charge and molecular weight).
Also Read: Electrochemistry
The electrolytes are often held as gel, such as agar-agar. The concentration of the salt solution and the diameter of the glass tube plays an important role in conductivity. Lowering the concentration and the diameter of the tube decreases the conductivity.
Filter Paper Bridge
They are another most commonly used bridge, consisting of filter paper or porous material soaked in electrolyte. Here, sodium chloride (NaCl) or potassium chloride (KCl) are commonly used electrolytes. Electrolytic concentration, porosity, and roughness of filter paper affect the conductivity. For higher conductivity, filter paper with smooth absorbent is used; they yield higher conductivity than rough paper with lower absorbent.
As stated above, a salt bridge’s main function is to maintain electrical neutrality between two beakers. To do so, the salt used must be inert. The ions need to move to and forth between the two half-cells. Unlike other salts, potassium chloride (KCl) and potassium nitrate (KNO3) are better inert salts. An inert salt is used to prevent the reactions from occurring between the salt and solution. The inert salt potassium chloride (KCl) is a commonly used salt because the potassium and chloride ions have a very common diffusion coefficient and minimising junction potential, but potassium chloride is unwise to use as an electrolyte when the electrode used is lead or silver because they form a precipitate.
Salt Bridge in Electrolysis
Like in the electrochemical cell, salt bridges have the same function in electrolytic cells too. If we immerse the two electrodes in one solution in a single container, no salt bridge is necessary, but if we want each electrode in a different solution and in separate containers, we need a salt bridge to complete the circuit. It contains mobile ions that act as charge carriers.
Preparing a Salt Bridge
Soaking String, Cotton, or Paper Material in an Electrolyte Solution
For preparing a bridge, take material that is large enough to reach two beakers. Place this material in a pool of electrolytes until they get saturated with the solution. Take the material from the electrolytic solution carefully and remove the excess amount of electrolyte.
Preparing Gel to Function as Bridge
The gel is suspended in an electrolytic solution, and they are treated with a buffered solution and heated later. The viscous gel is allowed to be set on a glass plate or tube.
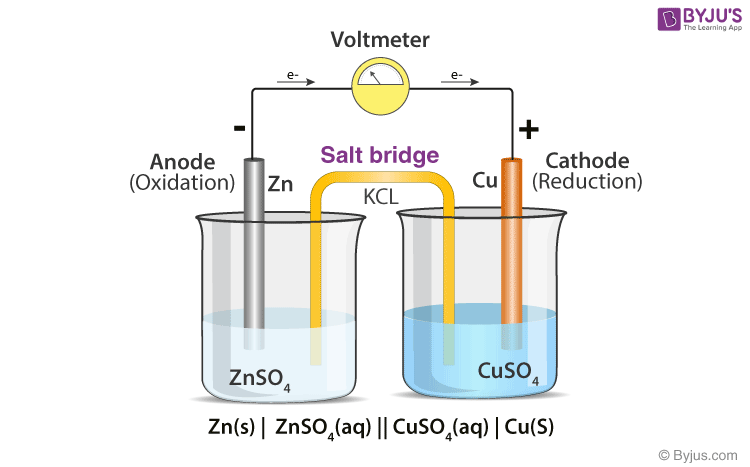
Working of a Salt Bridge
The oxidations that occur in an anode generate electrons and positive ions. Now, the electrons flow through the wire, leaving the unbalanced positive charge in a beaker. In order to maintain electrical neutrality, the negatively charged (NO3–) ion moves to the positively charged beaker (anodic half-cell).

A similar situation develops in the cathode cell but in reverse. Here, the Cu2+ ions are consumed. So, to maintain electrical neutrality, the K+ ions are migrated into this half-cell from the salt bridge. Hence, the electrical neutrality of the solution is maintained using the salt bridge.
What Happens if No Salt Bridge Is Used in a Galvanic Cell?
A galvanic cell is one where electricity is generated by a redox reaction. A salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt bridge present in the galvanic cell.
- Before wires are connected, the solution in each beaker is neutral.
- So, they have an equal number of positive charges and negative charges.
- The zinc bar (anode used in a galvanic cell) will give up to 2 electrons so that the electrons flow to the copper bar (cathode used in the galvanic cell) through the wire.
- So, the solution gains a positive charge since zinc loses electrons.
- The copper bar (cathode) takes the two electrons causing one positive copper ion (Cu+2) to leave the solution and accept the two electrons.
- When this happens, an atom of copper will deposit on the copper bar.
- Hence, this solution becomes negatively charged.
Now, there exist two voltages, one between the electrodes (metal bar) and the other between the charged solutions. The voltage between the metal bar or electrode is positive, and the voltage between the charged solution is negative. So, these voltages will cancel out, and no current will flow. This proves the importance of salt bridges in any electrochemical cell or electrolysis.

Comments