Acid and base are important topics that we come across or study in Chemistry. Acid and base are two major classifications of substances which include chemicals used in labs as well as substances that are found in our everyday life. Additionally, learning about these concepts is important from an examination point of view. Here we will discuss what acids and bases are, and look at their different characteristics and properties.
Robert Boyle’s Definition of Acid and Base
What does it actually mean for substances to be acidic or basic? Robert Boyle defined,
a) Acid as,
1) Having a sour taste
2) Being corrosive
3) Chemical that changes the colour of certain vegetable dyes, such as litmus.
Based on their occurrence, they are divided into two types – natural and mineral acids.
Natural Acids: These are obtained from natural sources, such as fruits and animal products.
For example, lactic, citric, tartaric acid, etc.
Mineral Acids: Mineral acids are acids prepared from minerals.
For example, hydrochloric acid (HCl), sulphuric Acid (H2SO4), nitric acid (HNO3), etc.
b) Base as,
1) Being slippery
2) Changing the colour of litmus from red to blue.
Arrhenius’s Definition of Acids and Bases
According to Arrhenius,
Acid
Acid is a substance that ionizes in water to give H+ ions
Hydrogen chloride ionizes in water to give hydrogen (H+).
Depending on the number of protons the acid gives, it can be classified as mono, di, or tribasic acid.
Monobasic acids: HCl, nitric acid, acetic acid
Dibasic acids: Sulphuric acid, phosphorous acid
Tribasic acids: Phosphoric acid
Base
A base is a substance that ionizes in water to give OH– ions.
NaOH dissociates in water to give the hydroxide (OH–).
Based on the number of available hydroxide ions, a base can be mono, di, or tri acidic bases.
Mono basic: NaOH, NH4OH
Dibasic: Ca(OH)2, Zn(OH)2
Tribasic: Fe(OH)3, Al(OH)3
This theory explains the similarity of properties among acids and bases, neutralization of bases by acid and vice versa. Acids provide the H+ ion, bases provide the OH– ion, and these ions combine to form water.
H+(aq) + OH–(aq) → H2O(l)
Acids and bases that ionize completely, giving larger hydrogen or hydroxide ions in solutions, are called strong acids and bases. HCl, HNO3, H2SO4, HCIO4, etc., are examples of strong acids, and NaOH, KOH, (CH3)4NOH, etc., are strong bases.
Acids and bases that are dissociated to a limited extent giving a lesser amount of hydrogen or hydroxide ions in solutions, are termed weak acids and bases.
Compounds containing ionizable hydrogen or hydroxide ions can be acid or base. CH4 is not an acid. Similarly, CH3OH, C2H5OH, etc., having OH groups, are not bases.
Disadvantages of Arrhenius Theory of Acid and Base
i) Applicable to aqueous solutions only, as acids and bases are defined from ionization in water.
ii) Cannot explain the acidic nature of nonmetal oxides, aluminium chloride and the basic nature of ammonia, sodium carbonate, and metal oxides.
Relative Strength of Acids and Bases
All strong acids and bases are equally ionized, and water is amphoteric. So, they have the same acidic or basic strength in water. This is known as the levelling effect.
The strength of acids also depends upon the solvent. Acetic acid is not receptive to taking up protons and has to be forced. Thus, acids HCIO4, H2S04, HCl and HN03, which have nearly the same strength in water, follow the order of HClO4> H2SO4 > HCl > HNO3 in acetic acid.
The real strength of acids can be judged by solvents. On the basis of proton interaction, solvents can be classified into four types:
- Protophilic solvents: Solvents that have a greater tendency to accept protons, for example, water, alcohol, liquid ammonia, etc.
- Protogenic solvents: Solvents that have the tendency to produce protons, for example, water, liquid hydrogen chloride, glacial acetic acid, etc.
- Amphiprotic solvents: Solvents that act both as protophilic or protogenic, for example, water, ammonia, ethyl alcohol, etc.
- Aprotic solvents: Solvents which neither donate nor accept protons, for example, benzene, carbon tetrachloride, carbon disulphide, etc.
HCl acts as an acid in the water, stronger acid in NH3, weak acid in CH3COOH, neutral in C6H6 and a weak base in HF.
Bronsted-Lowry Concept of Acid and Base
Bronsted acids are hydrogen-ion or proton donors.
Bronsted bases are hydrogen-ion or proton acceptors.
HCl donates H+ ion to a water molecule to form H3O+.
HCl acts as an acid, and water is a base.
Neutralization, as per the Bronsted model, involves the transfer of an H+ ion from an acid to a base. Acids can be
i) Neutral molecules.
ii) Positive ions
iii) negative ions.
Conjugate Acid-Base Pairs
In the reaction–
HCl and Cl– differ by a proton, and so are the NH3 and NH4+
NH4+, like HCl, can donate a proton and hence an acid. Since it has come from the base ammonia, it is called a conjugate acid of base ammonia.
Similarly, Cl–, like ammonia, can accept a proton and hence a base. It is considered as the conjugate base of the acid HCl.
The Bronsted-Lowry transfer can be written as,
Or as Acid + Base → Conjugate acid + Conjugate base
Conjugate pairs
A conjugate acid contains one more H atom and +charge than the base that formed it.
A conjugate base contains one less H atom and one more negative charge than the acid that forms it.
The conjugates will always be listed on the product side of the reaction.
Strong acid forms a weak conjugate base and vice versa.
Reactions always go from strong acid/base to weak acid/base.
Acid-Base Nature of Compounds
1. Compounds that contain hydrogen(+1) bound to a nonmetal are called nonmetal hydrides, and they are acidic.
2. Metal hydrides contain hydrogen (-1) bound to a metal. They give the H– (or hydride) ion.
The H– ion, with its pair of valence electrons, can abstract an H+ ion from a water molecule and increase OH– ion concentration. So, in a solution, metal hydrides are bases.
3. Nonmetal oxides dissolve in water to form acids. CO2 dissolves in water to give carbonic acid,
4. Metal oxides containing O2- ion reacts with water to give a pair of OH– ions and bases.
Metal oxides, therefore, fit the operational definition of a base.
5. Metal hydroxides, such as LiOH, NaOH, KOH, and Ca(OH)2, are bases.
The difference between the electronegativities of sodium and oxygen is very large ( ![]() EN = 2.5). As a result, the electrons in the Na-O bond are not shared equally; these electrons are drawn towards the more electronegative oxygen atom. NaOH, therefore, dissociates to give Na+ and OH– ions when it dissolves in water.
EN = 2.5). As a result, the electrons in the Na-O bond are not shared equally; these electrons are drawn towards the more electronegative oxygen atom. NaOH, therefore, dissociates to give Na+ and OH– ions when it dissolves in water.
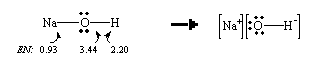
6. Nonmetal hydroxides, like hypochlorous acid (HOCl), HONO2, O2S(OH)2, and OP(OH)3, are
acids.
![]()
Here, the difference in electronegativities of the chlorine and oxygen atoms is small ( ![]() EN = 0.28). So, the electrons in the Cl-O bond are shared more or less equally by the two atoms. The O-H bond, on the other hand, is polar (
EN = 0.28). So, the electrons in the Cl-O bond are shared more or less equally by the two atoms. The O-H bond, on the other hand, is polar ( ![]() EN = 1.24). The electrons in this bond are drawn towards the more electronegative oxygen atom to form OCl–, and H+ ions are formed.
EN = 1.24). The electrons in this bond are drawn towards the more electronegative oxygen atom to form OCl–, and H+ ions are formed.
The acidic hydrogen atoms in the non-metal hydroxides aren’t bound to the nitrogen, sulfur, or phosphorus atoms but only to the oxygen atom. These compounds are oxyacids.
 |
 |
 |
 |
 |
 |
 |
 |
7. Oxides and hydroxides that lie between the metal and nonmetal oxides, or metal and nonmetal hydroxides, are amphoteric. The compounds, such as Al2O3 and Al(OH)3, can act as either acids or bases. Al(OH)3, for example, acts as an acid when it reacts with a base.
Conversely, it acts as a base when it reacts with an acid.
Lewis ( Electron) Concept of Acid-Bases
Species that can accept electron pairs are called Lewis acids.
- Molecules having a central atom with an incomplete octet (less than 8 electrons): BF3, BCl3, AlCl3, MgCl2. BeCl2. etc.
- Molecules having a central atom with empty d-orbitals: SiX4, GeX4, TiCl4, SnX4, PX3, PF5, SF4, SeF4, TeCl4, etc.,
- Molecules having multiple bonds between atoms of dissimilar electronegativity: CO2, SO2 and SO3. Under the influence of attacking Lewis base, one -electron pair will be shifted towards the more negative atom.
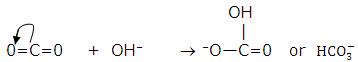
4. Simple cations: Cations that have a greater tendency to accept electrons: H+ and Ag+.
The following species can act as Lewis bases.
- Neutral species having at least one lone pair of electrons:

- Negatively charged species or anions: For example, chloride, cyanide, hydroxide ions, etc.
- It may be noted that all Bronsted bases are also Lewis bases, but all Bronsted acids are not Lewis acids.
The following compounds, for example, contain nonbonding pairs of electrons.
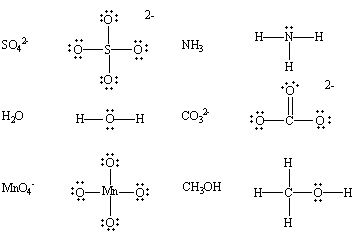
Related Topics
- Chemical Equilibrium
- Ionic Equilibrium – Degree of Ionization and Dissociation
- Equilibrium Constant – Characteristics and Applications
- Le Chatelier’s Principle on Equilibrium
- Solubility and Solubility Product
- pH Scale and Acidity
- pH and Solutions
- Hydrolysis, Salts, and Types
- Buffer Solutions

Comments