NCERT Solutions for Class 12 Chemistry Chapter 14 – Free PDF Download
*According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 10.
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules are intended for students of Class 12 to write their Class 12 Chemistry board examination successfully. The NCERT Solutions for Class 12 Chemistry deals with the topic of Biomolecules and contains questions and solutions on topics such as saccharides, glycogen, cellulose, starch and more. To become well-versed in these topics, access these solutions.
The NCERT Solutions for Class 12 Chemistry is developed by expert subject teachers according to the latest CBSE Syllabus 2023-24 and its guidelines. These solutions are written in simple language for ease of comprehension. Get the free PDF of NCERT Solutions for Class 12 Chemistry Chapter 14 from the link below.
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules
Class 12 Chemistry NCERT Solutions Chapter 14 Biomolecules – Important Questions
Q 1. What are monosaccharides?
Ans:
Monosaccharides, known as simple sugars, comprise one sugar unit that cannot be further broken down into simple sugars.
We can classify a monosaccharide on the basis of the number of carbon atoms and the functional group present in them. The monosaccharide, which contains an aldehyde group, is termed an aldose, and those which have a keto group are called ketoses. Depending on the number of carbon atoms present in a monosaccharide, it is further classified as trioses, tetroses, pentoses, hexoses and heptoses. For example, we can call an aldose which contains 3 carbon atoms as aldotriose and a keto which contains 3 carbon atoms as ketotriose.
Q 2. What are reducing sugars?
Ans:
Those type of carbohydrates which reduces Fehling’s solution and Tollen’s reagent are termed as reducing sugars.
Q 3. Write two main functions of carbohydrates in plants.
Ans:
The two main functions of carbohydrates in a plant are as follows:
(a) Polysaccharides like starch act as storage molecules.
(b) Cellulose is used to build the cell wall, and it is a polysaccharide.
Q 4. Classify the following into monosaccharides and disaccharides.
Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ans:
Monosaccharides: 2-deoxyribose, galactose, ribose, fructose
Disaccharides: lactose, maltose
Q 5. What do you understand by the term glycosidic linkage?
Ans:
The linkage which forms by the loss of water between two monosaccharide units through an oxygen atom is known as glycosidic linkage.
For example, in a sucrose molecule, two monosaccharide units,
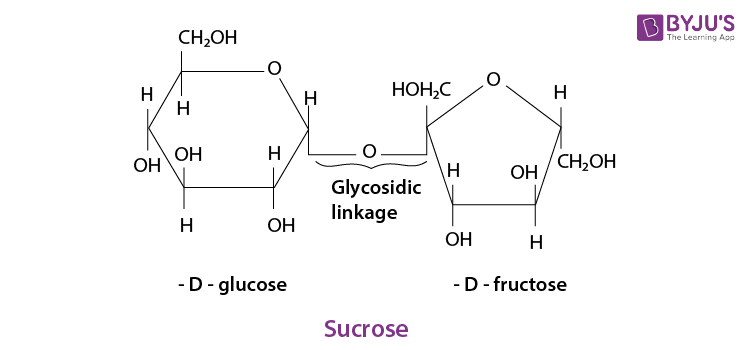
Q 6. What is glycogen? How is it different from starch?
Ans:
Glycogen, also termed animal starch, is found only in animals. It is a polysaccharide.
Both glycogen and starch are the main sources of glucose that provides energy to humans that are later converted into carbohydrates.
They differ in structure. Starch comprises a chain and a branched compound, whereas glycogen is composed of a single molecule and is branched.
Q 7. What are the hydrolysis products of (i) sucrose and (ii) lactose?
Ans:
(i) The hydrolysis of sucrose will give one molecule of
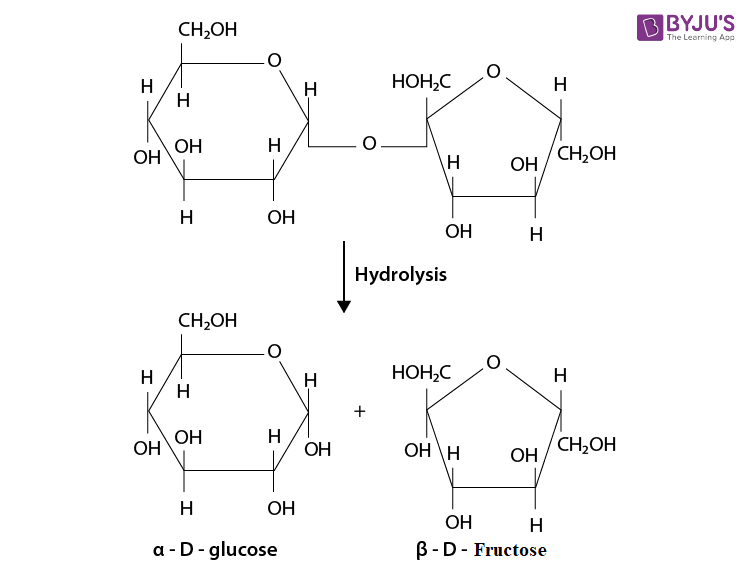
(ii) On hydrolysis of lactose, it will give
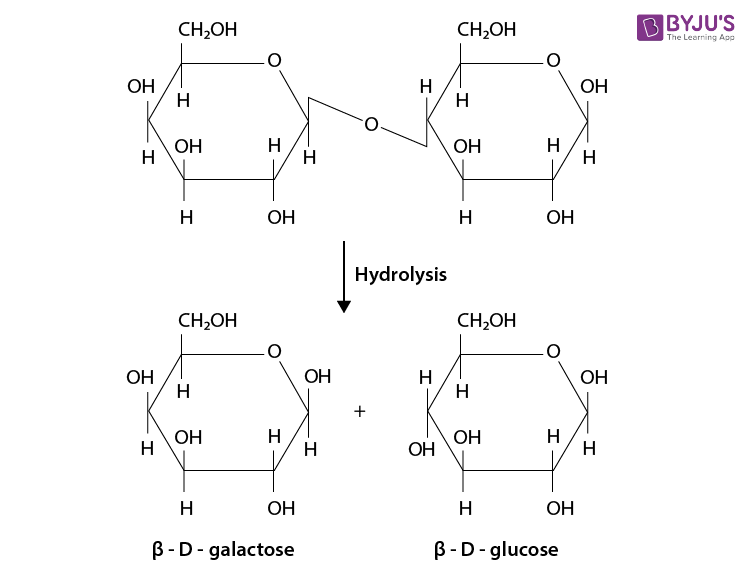
Q 8. What is the basic structural difference between starch and cellulose?
Ans:
Starch consists of two components – amylopectin and amylose. Amylose have a longer linear chain of
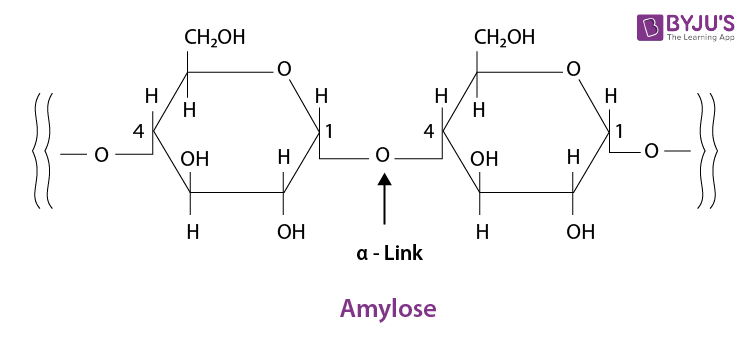
While amylopectin is a branched-chain polymer of
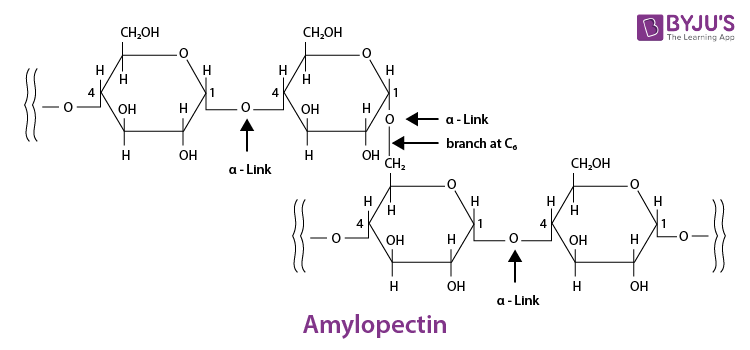
The cellulose is a straight-chain polysaccharide of
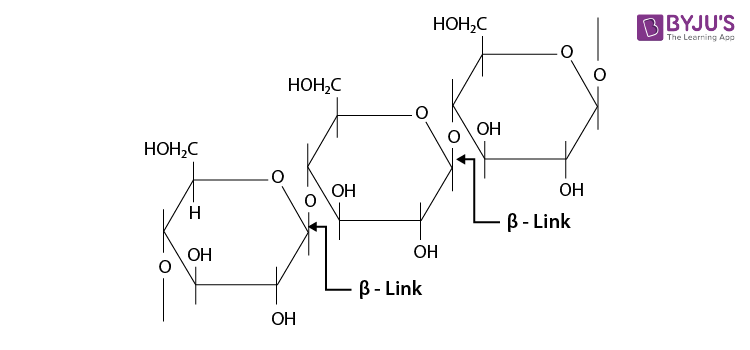
Q 9. What happens when D-glucose is treated with the following reagents?
(i) HI (ii) Bromine water (iii) HNO3
Ans:
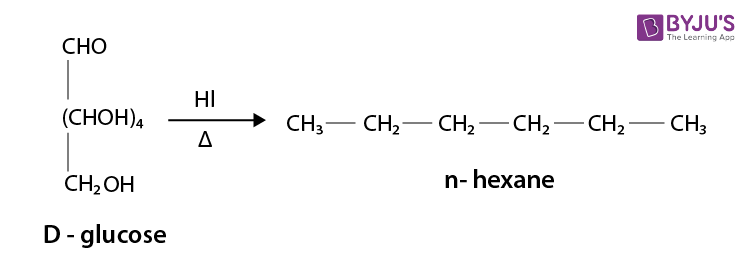
(i) After heating a D-glucose with HI for a long time, n-hexane is formed.
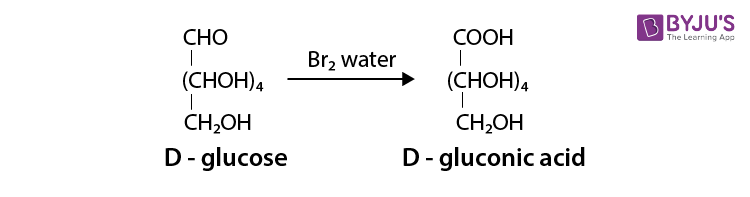
(ii) After treating a D-glucose with
(iii) After treating with
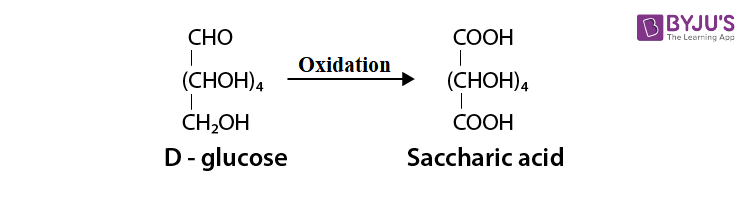
Q 10. Enumerate the reactions of D-glucose, which cannot be explained by its open chain structure.
Ans:
(i) The pentaacetate of glucose does not react with hydroxylamine. This shows that a free −CHO group is not present in glucose.
(ii) Aldehydes form the hydrogen sulphite additional product by giving 2,4 – DNP test, Schiff’s test and react with
(iii) Glucose is available in two crystalline forms
Q 11. What are essential and non-essential amino acids? Give two examples of each type.
Ans:
The amino acids, which are required by the human body, are called essential amino acids, but these cannot be produced inside the human body. They must be taken from any external source like food, for example, leucine and valine.
The amino acids which are required by the human body and can be produced inside the body are called non–essential amino acids, for example, glycine and alanine.
Q 12. Define the following as related to proteins
(I) Primary structure, (ii) Peptide linkage, (iii) Denaturation.
Ans:
(i) Primary Structure
We can refer to the specific sequence in which various amino acids are present if we talk about the primary structure of the protein, like the sequence of linkage between amino acids in a polypeptide chain.
The amino acids are arranged in a different sequence in each protein. A little difference in the sequence of the arrangements will create a completely different protein.
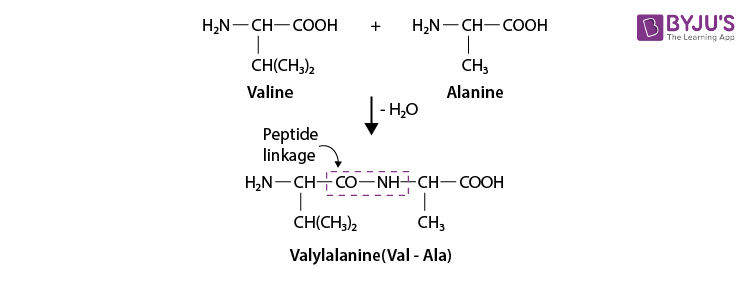
(ii) Peptide Linkage
A peptide linkage is an amide which is formed by the elimination of a water molecule between the –COOH group of one molecule of amino acid and
(iii) Denaturation
A protein has a unique 3-dimensional structure and a unique biological activity inside a biological system. In these types of circumstances, proteins are called native proteins. Whenever we put a native protein into a physical change like a change in temperature or any chemical change like a change in pH, then there its H-bonds are disturbed or changed.
This result in the unfolding of the globules ad uncoils the helix. And the consequences of this change are that the protein results in the loss of its biological activity. This loss of biological activity by the protein is called denaturation. During this process, no changes are encountered in the primary structure, whereas tertiary and secondary structures will be destroyed.
An example of denaturation proteins is the coagulation of egg white when an egg is boiled.
Q 13. What are the common types of the secondary structure of proteins?
Ans:
Secondary structures of proteins are of two types:
(a)
(b)
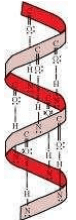
In this structure, the −NH group of an amino acid residue forms an H-bond with the group of the adjacent turn of the right–handed screw (
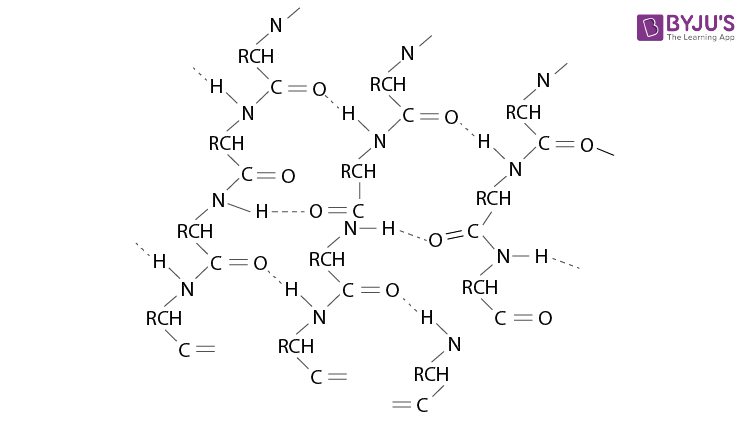
This structure is called so because it looks like the pleated folds of drapery. In this structure, the peptide chains are laid side by side after stretching out near the maximum extension. The intermolecular hydrogen bond keeps the peptide chain together.
Q 14. What type of bonding helps in stabilising the α-helix structure of proteins?
Ans:
The H-bonds formed between the −NH group of each amino acid residue and the group of the adjacent turns of the
Q 15. Differentiate between globular and fibrous proteins.
Ans:
| Globular Protein | Fibrous Protein | ||
| 1. | The polypeptide chain in this protein is folded around itself, giving rise to a spherical structure. | 1 | It is a fibre-like structure formed by the polypeptide chain. These are the proteins which are held together by strong hydrogen and disulphide bonds. |
| 2. | It is usually soluble in water. | 2. | It is usually not soluble in water. |
| 3. | Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair, collagen in tendons, and myosin in muscles. | 3. | All enzymes are globular proteins. Some hormones, such as insulin, are also globular proteins. |
Q 16. How do you explain the amphoteric behaviour of amino acids?
Ans:
In the presence of water or an aqueous solution, the carboxyl group of an amino acid can lose a proton, and the amino group can accept a proton to give a dipolar ion known as a zwitterion.
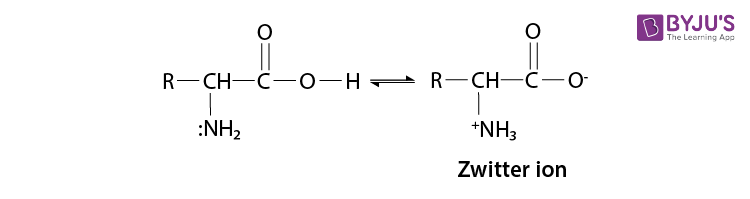
Therefore, the amino acid can act both as an acid and as a base in the presence of zwitterionic form.
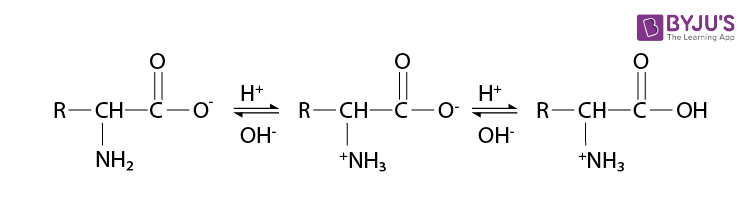
So, the amino acid show amphoteric behaviour.
Q 17. What are enzymes?
Ans:
The protein that catalyses the biological reactions are called enzymes. They are very particular in nature, and for some specific substrates, they catalyse particular reactions.
The enzymes are named after a particular reaction or, in common bases, they are named after a particular class of substrate.
For example, maltase is the enzyme which is used to catalyse the hydrolysis of maltose into glucose.
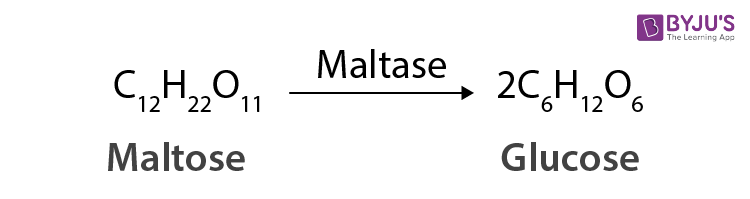
Also, oxidoreductase enzymes are those which are used to catalyse the oxidation of one substrate with the simultaneous reaction of another substrate.
The name of an enzyme ends with “-ase”
Q 18. What is the effect of denaturation on the structure of proteins?
Ans:
The outcome of denaturation, helixes get uncoiled, and globules get unfolded. There would be no change in the primary structure of the protein, while the secondary and the tertiary structure gets destroyed. We can say that the secondary and the tertiary–structured proteins are changed into primary–structured proteins. Also, because of the loss of secondary and tertiary structure, the enzymes lose their activity.
Q 19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Ans:
We can classify vitamins on the basis of solubility in water or fat into two categories.
(a) Water-soluble vitamins: Vitamins which are soluble in water come in the category.
For example, B group vitamins (
(b) Fat-soluble vitamins: Those vitamins which are soluble only in fat, not in the water, come under this group. For example, Vitamins A, D, E, and K.
However, biotin or Vitamin H is neither soluble in water nor in fat.
The vitamin which is responsible for the coagulation of blood is Vitamin K.
Q 20. Why are Vitamin A and Vitamin C essential to us? Give their important sources.
Ans:
These two vitamins are essential to us because the deficiency of these two vitamins causes us harmful diseases like xerophthalmia (which hardens the cornea of the eye) and night blindness. While the deficiency of Vitamin C causes scurvy (bleeding gums).
The sources of these two vitamins are given below:
Vitamin A: Carrots, fish liver oil, milk and butter.
Vitamin C: Amla, citrus fruits and green leafy vegetables.
Q 21. What are nucleic acids? Mention their two important functions.
Ans:
It is a molecule which is found as one of the constituents of chromosomes which is found in the nuclei of all living cells.
Nucleic acid can be categorised into two categories: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
Nucleic acids are long-chain polymers of nucleotides, so they are also known as polynucleotides.
(i) It is responsible for heredity. In heredity, there is a transfer of inherent characters from one generation to another. This process is held by the DNA.
(ii) The protein cell synthesis is held by nucleic acid (both RNA and DNA). Protein synthesis is majorly done by the various RNA molecules in a cell, while DNA contains the message for the synthesis of a specific protein.
Q 22. What is the difference between a nucleoside and a nucleotide?
Ans:
A nucleotide is formed by the combination of all three basic components of nucleic acids, i.e. base, pentose sugar, and phosphoric acid).
Therefore, Nucleotide = Base + Sugar + Phosphoric acid
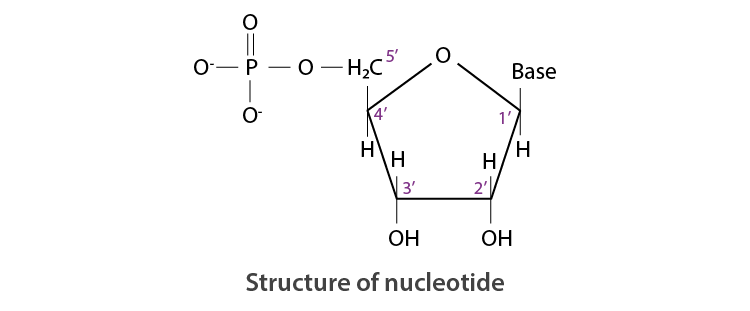
On the other hand, a nucleoside is formed by the attachment of a base to the 1’ position of the sugar.
Nucleoside = Sugar + Base
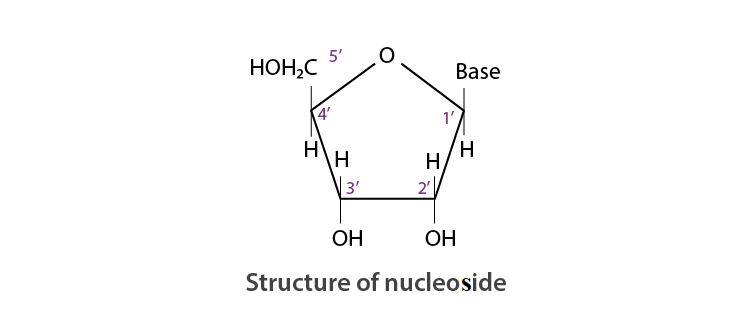
Q 23. The two strands in DNA are not identical but complementary. Explain.
Ans:
In the helical structure of DNA, the hydrogen bond holds the two strands between specific pairs of bases. Adenine forms a hydrogen bond with thymine, while cytosine forms a hydrogen bond with guanine. As a result, the two strands act as complementary to each other.
Q 24. Write the important structural and functional differences between DNA and RNA.
Ans:
The difference between DNA and RNA, on the basis of their functions, is as follows:
| DNA | RNA | ||
| 1 | DNA is the chemical basis of heredity. | 1 | RNA is not responsible for heredity. |
The differences on the basis of their structures are as follows:
| DNA | RNA | ||
| 1 | The sugar moiety in DNA molecules is \(\begin{array}{l}\beta\end{array} \) -D-2 deoxyribose. |
1 | The sugar moiety in RNA molecules is \(\begin{array}{l}\beta\end{array} \) -D-ribose. |
| 2 | Bases are Adenine(A), Guanine(G), Cytosine(C) and Thymine(T). | 2 | The bases are Adenine(A), Guanine(G), Cytosine(C), and Uracil(U). |
| 3 | The helical structure of DNA is double-stranded. | 3 | The helical structure of RNA is single-stranded. |
Q 25.
What are the different types of RNA found in the cell?
Answer
(i) Messenger RNA (m-RNA)
(ii) Ribosomal RNA (r-RNA)
(iii) Transfer RNA (t-RNA)
Biomolecules are the organic compounds present as essential constituents of the living organism in different cells. Biomolecules include carbohydrates, proteins, enzymes, vitamins and nucleic acids. To help students to get familiar with the topics, we have provided a downloadable form of solutions for Chapter 14 in PDF. This PDF will help students to score good marks. The biomolecules solutions PDF provided here has answers to the textbook questions along with other Chemistry Class 12 Important Questions, worksheets, MCQs, HOTS (Higher Order Thinking Skills) and exemplary questions prepared by experts at BYJU’S.
Class 12 NCERT Solutions for Chemistry Chapter 14 Biomolecules
Life is made up of chemicals, living beings are constituted of chemicals known as biomolecules, such as carbohydrates, vitamins, lipids, proteins and nucleic acids. These biomolecules interact with each other and constitute the molecular logic of life processes. In addition, some simple molecules like vitamins and mineral salts also play an important role in the functions of organisms. The structures and functions of some of these biomolecules are discussed in NCERT Solutions for Class 12 under Chemistry Chapter 14.
Class 12 Chemistry Chapter 14 Biomolecules is formulated as per the CBSE Syllabus for 2023-24. After studying this chapter, students will be able to explain the characteristics of biomolecules like carbohydrates, proteins and nucleic acids and hormones; classify carbohydrates, proteins, nucleic acids and vitamins on the basis of their structures; explain the difference between DNA and RNA and describe the role of biomolecules in bio-system.
Subtopics of Class 12 Chemistry Chapter 14 – Biomolecules
- Carbohydrates
- Proteins
- Enzymes
- Vitamins
- Nucleic Acids
- Hormones
The NCERT Chemistry Solutions for Class 12 Chemistry have been designed in such a way that they enable students to tackle chemistry questions effectively. Using these solutions, students can understand difficult concepts and perform better in the board exams. They can use NCERT Solutions for Class 12 Chemistry Chapter 14 PDF for their reference and be well-versed in all the topics before the board exam. Students can perform well in the board exams by going through and solving the NCERT Solutions. The solutions, which include important questions and sample papers, will help them get a broader idea about the different types of questions along with their difficulty level as well as the marking scheme.
Why Opt for BYJU’S?
BYJU’S NCERT Solutions for Chemistry covers all the chapters in general. So, students can benefit from not just one chapter but all the chapters’ NCERT Solutions for Class 12 Chemistry. Students also get an additional advantage as they can learn NCERT or CBSE-based Class 12 Chemistry chapters and topics within their comfort zone. They can access the materials right from their homes or from anywhere. Students can also download and use our free Learning App. BYJU’S also provides experienced subject experts who can clarify all their doubts while guiding them to learn the subject and its concepts in a more structured manner.
In case a student faces problems while referring to NCERT Class 12 Chemistry Solutions, they can approach the BYJU’S support team to clear all their doubts. Apart from Chemistry, students can also submit all their queries regarding other subjects, including Physics, Maths and Biology. And that is not just it. BYJU’S also keeps track of the progress that students make. Besides, feedback is provided at regular intervals.























Comments