NCERT Solutions for Class 12 Chemistry Chapter 16 – Free PDF Download
*According to the CBSE Syllabus 2023-24, this chapter has been removed.
NCERT Solutions for Class 12 Chemistry Chapter 16 Chemistry in Everyday Life are the best study materials that can help students to score good marks in the CBSE Class 12 examination. The NCERT Solutions for Class 12 Chemistry give them precise answers to the textbook questions, MCQs, HOTS (Higher Order Thinking Skills), worksheets, exercises and assignments.
To master Class 12 Chemistry, one must solve the questions provided at the end of each chapter. Solving these questions with the help of NCERT Solutions for Class 12 Chemistry is the best way. These solutions are framed in a simple step-wise manner for better understanding. Access the free PDF of the NCERT Solutions for Class 12 Chemistry for this chapter from the link attached below.
NCERT Solutions Class 12 Chemistry Chapter 16:-
Class 12 Chemistry NCERT Solutions Chapter 16 Chemistry in Everyday Life – Important Questions
Question 1:
Why do we need to classify drugs in different ways?
Answer :
The reason for the drug’s classification is as follows:
(i) The pharmacological impact is based on
To physicians, this definition is helpful. It provides a whole array of drugs to classify drugs for different diseases.
(ii) Based on the action on the drugs
This is based on a drug’s action on a specific biochemical process.
(iii) Building on chemical structure
The range of drugs that share common structural characteristics and have similar pharmacological activity.
(iv) Building on molecular objectives
Many medications have the same action function on targets. In such cases, this distinction is useful.
Question 2:
Explain the term, target molecules or drug targets, as used in medicinal chemistry.
Answer
The drug targets are the key molecule responsible for some metabolic pathways that can cause specific diseases. The drug targets are proteins, nucleic acids, carbohydrates and lipids.
Chemical agents used to block those target molecules are called drugs by fusing with the active sites of key molecules.
Question 3:
Name the macromolecules that are chosen as drug targets.
Answer
Carbohydrates, lipids, proteins and nucleic acids are the macromolecules selected as targets for the drugs.
Question 4:
Why should medicines not be taken without consulting doctors?
Answer
Medicines should not be taken without consulting a doctor because they can bind to more than one receptor site. Thus it can be harmful to some receptor sites. Medicines, when taken in higher doses, can cause harmful effects. So medicines can be poisonous.
Question 5:
Define the term chemotherapy.
Answer
Chemotherapy is the use of chemicals for medicinal effects. Sources are the use of chemical agents for disease prevention, diagnosis and treatment.
Question 6:
Which forces are involved in holding the drugs to the active site of enzymes?
Answer
The forces responsible are
(1) Hydrogen bonding
(2) Ionic bonding
(3) van der Waals force
(4) Dipole-dipole interaction
Question 7:
While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
Answer
Certain drugs only affect specific receptors. Antacids and antiallergic drugs do not interfere, as they work on different receptors. That is why antacids and antiallergic drugs interfere with histamine function but not with each other.
Question 8:
A low level of noradrenaline is the cause of depression. What type of drugs are needed to cure this problem? Name two drugs.
Answer
Antidepressant drugs are used to lessen the depression effect. These drugs contain enzymes that catalyse the degradation of the neurotransmitter noradrenaline. The neurotransmitter is, therefore, slowly metabolised and can activate the receptor for a longer period of time.
The two anti-depressant drugs are
1. Phenelzine
2. Iproniazid
Question 9:
What is meant by the term ‘broad spectrum antibiotics’? Explain.
Answer
Antibiotics are known as broad-spectrum antibiotics, which are effective against a wide range of gram-negative and gram-positive bacteria. E.g., Chloramphenicol.
This is used to treat acute fever, typhoid, meningitis, dysentery, tuberculosis and certain types of urinary tract infections. The other 2 broad-spectrum antibiotics are vancomycin and ofloxacin. Amoxicillin and ampicillin − synthetically derived from penicillin – are also antibiotics of broad-spectrum.
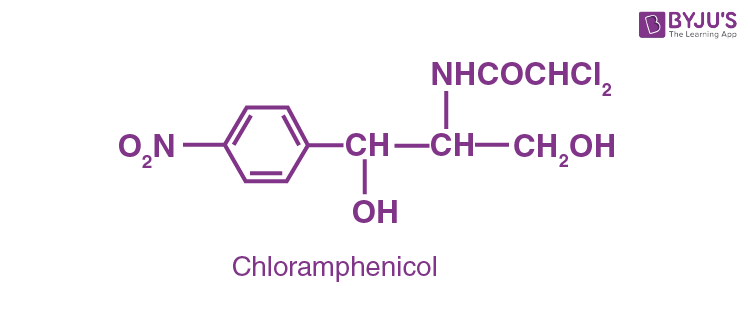
Question 10:
How do antiseptics differ from disinfectants? Give one example of each.
Answer
Disinfectants and antiseptics against microorganisms are really successful. Antiseptics are used to treat living tissues such as cuttings, wounds, diseased skin surfaces and ulcers, while disinfectants are used for objects such as floors, drainage systems, instruments, etc. Disinfectants damage living tissues.
Iodine is a potent antiseptic. The iodine tincture is applied to wounds. One per cent phenol solution is used as a disinfectant.
Question 11:
Why are cimetidine and ranitidine better antacids than sodium hydrogen carbonate or magnesium or aluminium hydroxide?
Answer
The antacids that neutralise excess hydrochloric acid in the stomach are magnesium hydroxide, sodium hydrogen carbonate, and aluminium hydroxide. However, the motive for releasing excess acid remains untreated.
Cimetidine and ranitidine are good antacids because they control acidity cause. These drugs prevent histamine from interacting with the receptors present in the walls of the stomach and can, therefore, reduce the amount of acid released by the stomach.
Question 12:
Name a substance which can be used as an antiseptic as well as a disinfectant.
Answer
Phenol is a substance which can be used as an antiseptic and as a disinfectant. 0.2 per cent phenol solution can be used as an antiseptic, and 1 per cent of the solution should be used as a disinfectant.
Question 13:
What are the main constituents of Dettol?
Answer
Dettol’s principal constituents are chloroxylenol and
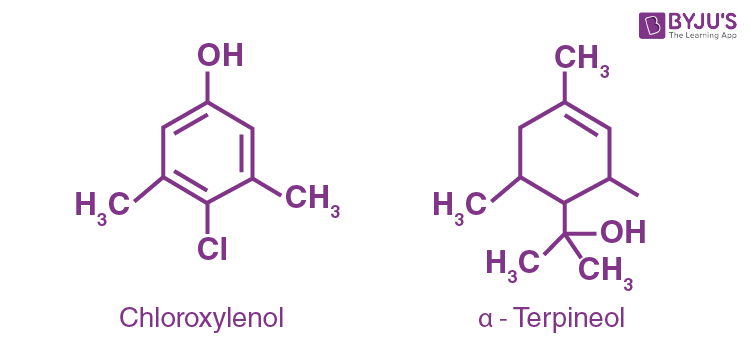
Question 14:
What is a tincture of iodine? What is its use?
Answer
For alcohol-water mixtures, 2-3 per cent of iodine is referred to as iodine tincture and is primarily added to wounds.
Question 15:
What are food preservatives?
Answer
Chemicals that prevent microbial growth are referred to as preservatives for food. They cut back on spoilage. Some food preservatives are sugar, table salt, vegetable oil, propanoic acid salts and sodium benzoate(C6H5COONa).
Question 16:
Why is the use of aspartame limited to cold foods and drinks?
Answer
Aspartame is not stable at cooking temperature, and therefore, its use is limited only to cold foods and beverages.
Question 17:
What are artificial sweetening agents? Give two examples.
Answer
Those chemicals which sweeten food are called artificial sweeteners. Artificial sweeteners don’t add calories to our bodies and don’t hurt the human body, either. Some known artificial sweeteners are sucralose, aspartame, alitame and saccharin.
Question 18:
Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Answer
Saccharin, aspartame and alitame are sweetening agents used to prepare sweets for patients with diabetes.
Question 19:
What problem arises in using alitame as an artificial sweetener?
Answer
Alitame is a sweetener of great potency. The sweetness of the food while using alitame as an artificial sweetener is difficult to control.
Question 20:
How are synthetic detergents better than soaps?
Answer
Synthetic detergents are used in both soft water and hard water, while soaps are used in soft water. In hard water, soaps aren’t effective. Synthetic detergents are, therefore, better than soaps.
Question 21:
Explain the following terms with suitable examples
(i) cationic detergents,
(ii) anionic detergents and
(iii) non-ionic detergents.
Answer
(i) Cationic detergents
Cationic detergents are commonly called quaternary ammonium salts of acetates, chlorides or bromides.
Cationic detergents are named as such because the cationic part of the aforementioned compound detergents has an extended hydrocarbon chain and a positive charge on the N atom.
Example: Cetyltrimethylammonium bromide
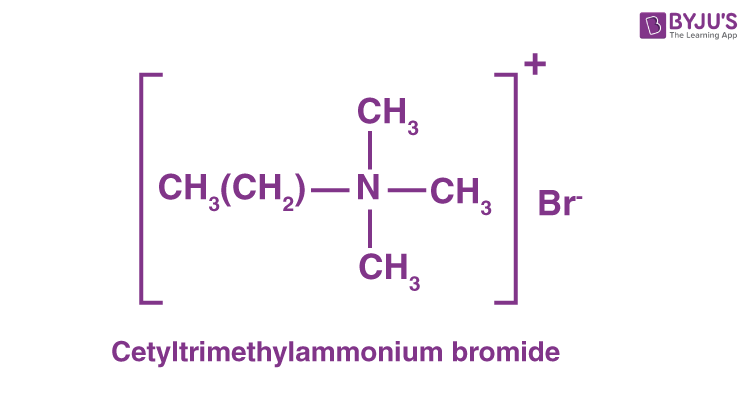
(ii) Anionic detergents
Type of anionic detergents are as follows:
1. Sodium alkyl sulfate detergents are simply long-chain sodium alcohol salts. They are made by using concentrated sulphuric acid to react to such alcohol and subsequently by sodium hydroxide.
Examples of such detergents include sodium lauryl sulfate (
2. Sodium alkylbenzene sulphonates: Sodium salts of long-chain alkyl benzene sulphonic acids are such detergents. These are synthesised by benzene alkylation by Friedel-Crafts, along with alkyl halides or alkenes with long chains. The resulting product initially reacts with concentrated sulphuric acid and subsequently reacts with sodium hydroxide. Sodium 4-(1-dodecy) benzenesulfonate is an example of an anionic detergent.
(iii) Non-ionic detergents
There are no ions in the molecules of those detergents. They are a good example of high-molecular-mass alcohol esters. These are prepared by the stearic acid and polyethylene glycol reactions.
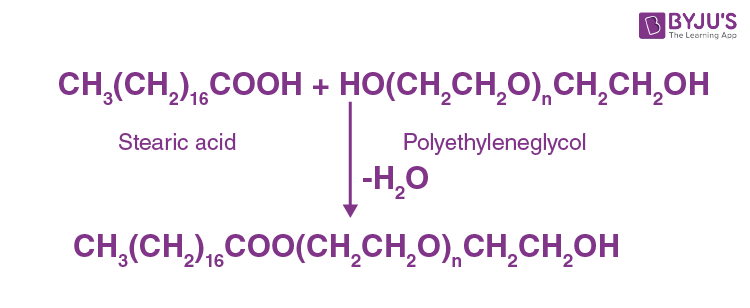
Question 22:
What are biodegradable and non-biodegradable detergents? Give one example of each.
Answer
Biodegradable detergents are Bacteria degrading detergents. They do have straight chains of hydrocarbons. Sodium lauryl sulfate, for example.
The non-biodegradable detergents are detergents that bacteria can not degrade. Their hydrocarbon chains are strongly branched.
For example, benzene sulphonate with sodium -4- (1, 3, 5, 7- tetramethyl octyl).
Question 23:
Why do soaps not work in hard water?
Answer
Sodium or potassium salts with long-chain fatty acids are present in soaps. Hard water contains magnesium and calcium. When the ions displace sodium or potassium on dissolving soaps in hard water, insoluble calcium or magnesium salts of fatty acids form. Separate those insoluble salts as scum.
This is why soaps do not work in harsh water.
Question 24:
Can you use soaps and synthetic detergents to check the hardness of water?
Answer
Soaps will precipitate in hard water, but in soft water, they will not get precipitated and can, therefore, be used to find the water’s hardness. On the other hand, synthetic detergents won’t get precipitated in both hard water and soft water and can’t be used to find water hardness.
Question 25:
Explain the cleansing action of soaps.
Answer
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. The dust particles are then easily rinsed away by water.
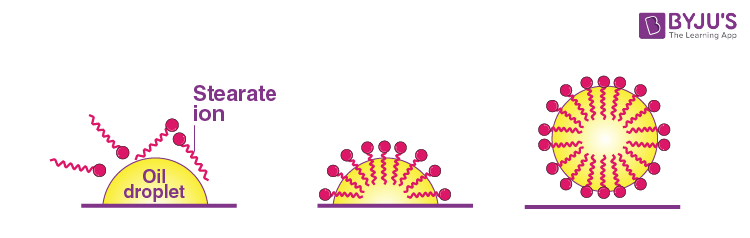
Question 26:
If the water contains dissolved calcium hydrogencarbonate, out of soaps and synthetic detergents, which one will you use for cleaning clothes?
Answer
Synthetic detergents are commonly used for clothes washing. Such ions form insoluble salts when dissolved in water containing calcium ions which are of no use. Synthetic detergents are dissolved in calcium ion-containing water, such ions form soluble salts which act as cleaning agents.
Question 27:
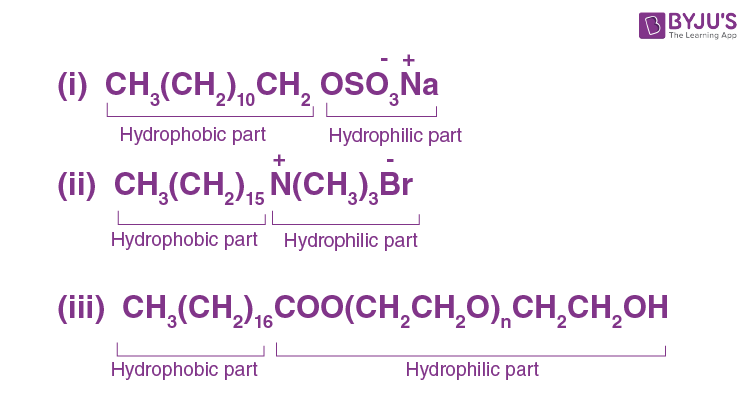
Label the hydrophilic and hydrophobic parts in the following compounds.
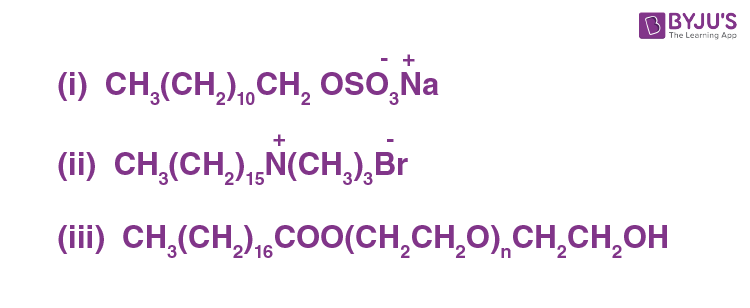
Answer

Chemistry influences every sphere of human life, and the principles of chemistry have been used for the benefit of humankind. Chemistry in everyday life questions and answers PDF are prepared by experienced teachers, as per the latest CBSE syllabus. Students must practise the NCERT Solutions for Class 12 Chemistry Chapter 16 regularly to excel in the board exam.
Class 12 NCERT Solutions for Chapter 16 Chemistry in Everyday Life
Class 12 Chemistry Chapter 16 includes the topic – Drugs and their classifications
- Drug Target Interaction: Enzymes as drug targets, Receptors as drug targets.
- Therapeutic Action of different classes of drugs: Antacids, Antihistamines, Neurologically Active Drugs, Antimicrobials and Antifertility Drugs.
- Chemicals in food: Artificial Sweetening Agents and Food Preservatives.
- Cleansing Agents: Soaps and Synthetic Detergents.
The most abundant metal found in the earth’s crust is Aluminium which is Approx 8.3% by weight. Cleaning of ore or removal of particles like sand or clay from the ore is called dressing of ore or concentration of ore. This is done in the following steps: Hydraulic washing, magnetic separation, froth floatation method, and leaching. This was brief on Chemistry in everyday life and can be well comprehended with the help of NCERT Solutions for Class 12. Students must also solve the exemplary questions of this chapter to attain a firm grip on the key concepts.
Subtopics of Class 12 Chemistry Chapter 16 – Chemistry in Everyday Life
- Drugs and Their Classification
- Drug-Target Interaction
- Therapeutic Action of Different Classes of Drugs
- Chemicals in Food
- Cleansing Agents
Why Opt for BYJU’S?
Chemistry is a tough subject for many students, and they always struggle to remember the concepts, derivations, and chemical and structural formulae. BYJU’S videos guide students in remembering the concepts and assignments provided by us, which will help them in practising important derivations, numerical problems, and structural formulas.
Apart from NCERT Solutions Class 12 Chemistry Chapter 16 – Chemistry in Everyday Life, students can access other NCERT Solutions for other chapters as well. Students can also download worksheets, assignments, NCERT Class 12 Chemistry PDF, and other study materials for exam preparation and to score better marks.
Frequently Asked Questions on NCERT Solutions for Class 12 Chemistry Chapter 16
Which topics are important in Chapter 16 of NCERT Solutions for Class 12 Chemistry?
1. Drugs and their Classification
2. Drug-Target Interaction
3. Therapeutic Action of Different Classes of Drugs
4. Chemicals in Food
5. Cleansing Agents
What questions can I expect from Chapter 16 of NCERT Solutions for Class 12 Chemistry?
1. Explain the term broad-spectrum antibiotics.
2. What is the tincture of iodine and explain its uses?
3. Which substance can be used as both an antiseptic and disinfectant?
4. Why does soap not give lather in hard water?
5. Why are synthetic detergents better than soaps?









Comments