According to the CBSE Syllabus 2023-24, this chapter has been removed from NCERT Class 12 Chemistry textbook.
The event of attracting and keeping the molecule of a substance on the surface of a solid is called adsorption. This results in an increased concentration on the surface than in the bulk. The expanse of adsorption of a gas on a solid material depends on the following factors: surface area of the solid, temperature of the gas, nature of the gas, the pressure of the gas, and nature of the solid.
- Adsorbate – The substance that is absorbed. It is held by weak van der Waals forces in physisorption, whereas in chemisorption, it is held by a strong chemical bond.
- Adsorbent – The substance on which adsorption occurs.
- Adsorption isotherm – It is defined as the relationship between the pressure of the gas and the extent of adsorption at a constant temperature.
CBSE Class 12 Chemistry Chapter 5 Surface Chemistry Related Links
- Catalysis
- Catalyst
- Catalysis – How does a catalyst work?
- Adsorption Theory Of Heterogeneous Catalyst
Types of Catalysis
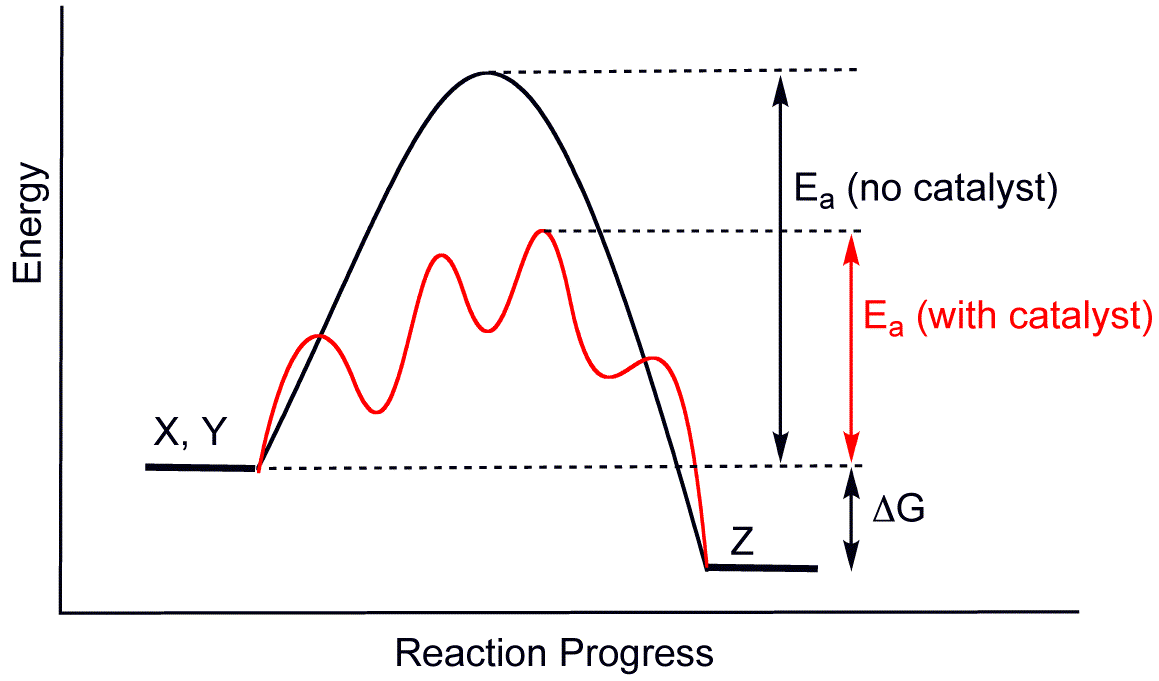
A substance that enhances the rate of a chemical reaction without getting itself consumed by the reaction is called a catalyst. It does not undergo any change and can be recovered in a chemically unchanged form. The event occurring with a catalyst is known as catalysis.
- Homogeneous catalysis – When the catalyst and reactants are in the same phase, it is called homogeneous catalysis.
- Heterogeneous catalysis – When the catalyst and reactants are in a different phase, it is called heterogeneous catalysis.
Few Important Questions
- Explain the difference between absorption and adsorption with an example.
- Define lyophobic and lyophilic sols with one example each. Also, explain which sol is easily coagulated and why?
- Explain how the emulsion is stabilized with emulsifiers. Name any two emulsifiers.
- What is aerosol? Write one example
- Define enzymes and explain enzyme catalysis mechanism.
| Also Access |
| NCERT Solutions for Class 12 Chemistry Chapter 5 |
| NCERT Exemplar for Class 12 Chemistry Chapter 5 |
To discover more about Surface Chemistry Class 12 Notes, register with BYJU’S.
For more questions on Surface Chemistry and Chemical Kinetics, watch the below video

Other Important Links:
| Difference Between Adsorption And Absorption | Catalyst |
Frequently Asked Questions on CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry
What is the definition of a catalyst?
Any substance that increases the rate of a reaction without itself being consumed is called a Catalyst.
What is chemical kinetics?
The branch of physical chemistry that is concerned with understanding the rates of chemical reactions.
What is the meaning of adsorption?
Adsorption is the sticking of atoms or molecules to a surface. This surface is known as an Adsorbent.
Comments