Science is a subject that explains how the world around us is made and the chemical reactions that make things happen around us. From rust to decomposition, chemical reactions provide a more in-depth insight into how molecular interaction and changes occur. Chapter 1 of CBSE class 10 Science explains how a substance changes form.
Learn more about chemical reactions and equations by exploring CBSE Notes for Class 10 Science Chapter 1. These CBSE notes are comprehensive and detailed yet concise enough to glance through for exam preparations.
CBSE Class 10 Science Revision Notes Chapter 1 Chemical Reactions and Equations
Chemical Reactions
A chemical reaction occurs when one or more reactants are changed into one or more products. The constituent atoms of the reactants are rearranged in a chemical reaction, resulting in the formation of various substances as products.
Physical and Chemical Changes
Chemical change – one or more new substances with new physical and chemical properties are formed.
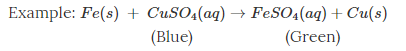
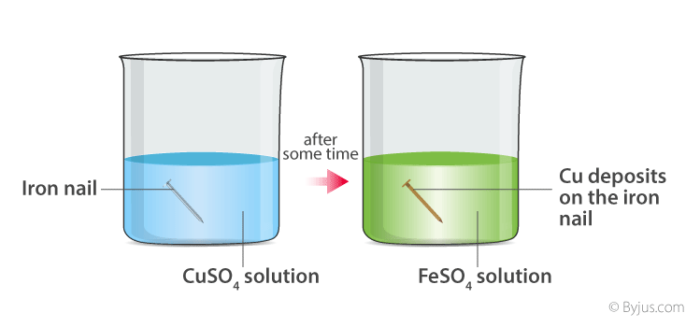
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper, are formed.
Physical change – change in colour or state occurs, but no new substance is formed.
Example: Water changes to steam on boiling, but no new substance is formed (Even though steam and water look different when they are made to react with a piece of Na, they react the same way and give the exact same products). This involves only a change in state (liquid to vapour).
To know more about Physical and Chemical Changes, visit here.
Students can refer to the short notes and MCQ questions along with separate solution pdf of this chapter for quick revision from the links below:
- Chemical Reactions and Equations Short Notes
- Chemical Reactions and Equations MCQ Practice Questions
- Chemical Reactions and Equations MCQ Practice Solutions
Observations that Help Determine a Chemical Reaction
A chemical reaction can be determined with the help of any of the following observations.
a) Evolution of a gas
b) Change in temperature
c) Formation of a precipitate
d) Change in colour
e) Change of state
Chemical Reaction
Chemical reactions are chemical changes in which reactants transform into products by making or breaking bonds (or both) between different atoms.
A chemical reaction is a process that causes one set of chemical components to change into another. Chemical reactions are defined as changes in the locations of electrons in the formation and breaking of chemical bonds between atoms, with no change in the nuclei, and are described using a chemical equation. At a given temperature and chemical concentration, chemical reactions occur at a predictable rate. Reaction speeds often increase as the temperature rises because more thermal energy is available to attain the activation energy required to break bonds between atoms.
Types of Chemical Reactions
Taking into consideration different factors, chemical reactions are grouped into multiple categories.
A few examples are:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
To know more about Chemical Reactions, visit here.
Chemical Reactions and Equations I
Word Equation
A word equation is a chemical reaction expressed in words rather than chemical formulas. It helps identify the reactants and products in a chemical reaction.
A chemical reaction is described using a word equation, which is a shorthand manner of expressing it. The names of the reactants are shown on the left side of a word equation. If there is more than one reactant, the names of the reactants are separated by a plus sign (+). Products are shown on the right side of a word equation. If there is more than one product, the names of the products are separated by a plus sign (+).
For example,
Sodium + Chlorine → Sodium chloride
The above equation means: “Sodium reacts with chlorine to form sodium chloride.”
Symbols of Elements and Their Valencies
A symbol is a chemical code for an element. Each element has a one or two-letter atomic symbol, which is, in most cases, the abbreviated form of its name.
Valency is the combining capacity of an element. It can be considered as the number of electrons lost, gained or shared by an atom when it combines with another atom to form a molecule.
Writing Chemical Equations
Representation of a chemical reaction in terms of symbols and chemical formulae of the reactants and products is known as a chemical equation.
![]()
• For solids, the symbol is “(s)”.
• For liquids, it is “(l)”.
• For gases, it is “(g)”.
• For aqueous solutions, it is “(aq)”.
• For gas produced in the reaction, it is represented by “(↑)”.
• For precipitate formed in the reaction, it is represented by “(↓)”.
To know more about Chemical Equations, visit here.
Balancing of a Chemical Reaction
Law of Conservation of Mass
According to the Law of Conservation of Mass, no atoms can be created or destroyed in a chemical reaction, so the number of atoms for each element on the reactants side has to balance the number of atoms that are present on the products side.
In other words, the total mass of the products formed in a chemical reaction is equal to the total mass of the reactants participating in a chemical reaction.
Balanced chemical equation
The chemical equation in which the number of atoms of each element on the reactants side is equal to that of the products side is called a balanced chemical equation.
To know more about Balanced Chemical Equations, visit here.
Steps for Balancing Chemical Equations
The changes that occur during a chemical reaction are represented by a chemical equation.
Reactants → Products
The equilibrium of all chemical equations must be maintained. This means that on both sides of the arrow, the number of each sort of atom must be the same.
Chemical equations are balanced using coefficients. A coefficient is a numerical value that is added to the front of a chemical symbol or formula. It indicates the number of atoms or molecules of the material involved in the process.
Place coefficients in front of symbols or formulas as needed to balance a chemical equation so that the same number of each type of atom appears in both reactants and products.
For example,
Zn + HCl → ZnCl2 + H2
The balanced equation is
Zn + 2HCl → ZnCl2 + H2
Hit and trial method: While balancing the equation, change the coefficients (the numbers in front of the compound or molecule) so that the number of atoms of each element is the same on each side of the chemical equation.
For more information on Steps for balancing chemical equations, watch the below video

Short-Cut Technique for Balancing a Chemical Equation
Example:
aCaCO3 + bH3PO4 → cCa3(PO4)2 + dH2CO3
Set up a series of simultaneous equations, one for each element.
Ca: a=3c
C: a=d
O: 3a+4b=8c+3d
H: 3b=2d
P: b=2c
Let’s set c=1
Then a=3 and
d = a = 3
b = 2c = 2
So a=3; b=2; c=1; d=3
The balanced equation is
3CaCO3 + 2H3PO4 → Ca3 (PO4)2 + 3H2CO3
To know more about the Balancing of a Chemical Equation, visit here.
Chemical Reactions and Equations II
Types of Chemical Reactions
Taking into consideration different factors, chemical reactions are grouped into multiple categories.
A few examples are:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
To know more about Types of Chemical Reactions, visit here.
Combination Reaction
In a combination reaction, two elements or one element and one compound or two compounds combine to give one single product.

When quicklime or calcium oxide (CaO) reacts with water, slaked lime [Ca(OH)2] is formed. During this reaction, a large amount of heat is released. So, this reaction is an exothermic Reaction.
CaO + H2O → Ca(OH)2
Decomposition Reaction
A single reactant decomposes on the application of heat or light, or electricity to give two or more products.
Types of decomposition reactions:
a. Decomposition reactions which require heat-thermolytic decomposition or thermolysis.

b. Decomposition reactions which require light-photolytic decomposition or photolysis.

c. Decomposition reactions which require electricity – electrolytic decomposition or electrolysis.
For more information on Decomposition Reaction, watch the below video

Displacement Reaction
A more reactive element displaces a less reactive element from its compound or solution.

Double Displacement Reaction or Precipitation Reaction
An exchange of ions between the reactants takes place to give new products.
For example, ![]()
An insoluble compound called precipitate forms when two solutions containing soluble salts are combined.
![]()
One of the best examples of precipitation reactions is the chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride is precipitated out. This is the insoluble salt formed as a product of the precipitation reaction. The chemical equation for this precipitation reaction is provided below.
AgNO3(aqueous) + KCl(aqueous) —–AgCl(precipitate) + KNO3(aqueous)
To know more about Precipitation Reactions, visit here.
Redox Reaction
A redox reaction occurs when the oxidation states of the substrate change. The loss of electrons or an increase in the oxidation state of a chemical or its atoms is referred to as oxidation. The gain of electrons or a decrease in the oxidation state of a chemical or its atoms is referred to as reduction.
Oxidation and reduction take place simultaneously.
Oxidation: Substance loses electrons or gains oxygen or loses hydrogen.
Reduction: Substance gains electrons or loses oxygen or gains hydrogen.
Oxidising agent – a substance that oxidises another substance and self-gets reduced.
Reducing agent – a substance that reduces another substance and self-gets oxidised.

To know more about Redox Reaction, visit here.
Endothermic and Exothermic reaction
Exothermic reaction – heat is evolved during a reaction. Most of the combination reactions are exothermic.
Al + Fe2O3 → Al2O3 + Fe + heat
CH4 + 2O2 → CO2 + 2H2O + heat
To know more about Exothermic Reactions, visit here.
Effect of Oxidation Reaction in Everyday Life
Endothermic – Heat is required to carry out the reaction.
6CO2 + 6H2O + Sunlight → C6H12O6 + 6O2
Glucose
Most of the decomposition reactions are endothermic.
To know more about Endothermic Reactions, visit here.
Corrosion
Gradual deterioration of a material, usually a metal, by the action of moisture, air or chemicals in the surrounding environment.
Rusting:
4Fe(s) + 3O2(from air) + xH2O(moisture) → 2Fe2O3.xH2O(rust)
Corrosion of copper:
Cu(s) + H2O(moisture) + CO2(from air) → CuCO3.Cu(OH)2(green)
Corrosion of silver:
Ag(s) + H2S (from air) → Ag2S(black) + H2(g)
To know more about Corrosion, visit here.
Rancidity
It refers to the oxidation of fats and oils in food that is kept for a long time. It gives foul smell and bad taste to food. Rancid food causes stomach infections during consumption.
Prevention:
(i) Use of air-tight containers
(ii) Packaging with nitrogen
(iii) Refrigeration
(iv) Addition of antioxidants or preservatives
Read more
- NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations
- Important Questions for Class 10 Science Chapter 1 – Chemical Reactions And Equations
- Acids, Bases and Salts Class 10 Chapter 2 Notes
- NCERT Exemplar Class 10 Science Solutions for Chapter 1 – Chemical Reactions And Equations
- Maths Notes For Class 10
- CBSE Class 10 Social Science Notes
Frequently Asked Questions on Chemical Reactions and Equations
Carbon reacts with oxygen to give carbon dioxide. This is an example of which type of reaction?
This is an example of a combination reaction since two reactants are combined to form a single product.
Identify the type of chemical reaction taking place when silver chloride turns black on exposure to sunlight.
It is a decomposition reaction that occurs in the presence of sunlight, and hence it is a photochemical decomposition reaction.
In electrolysis of water (acidified), what is the name of the gases that are evolved at the anode and cathode, respectively?
In the electrolysis of water (acidified), the gases that are evolved at the anode and cathode, respectively, are oxygen and hydrogen. Hydrogen ions gain electrons from the cathode and form hydrogen gas, and oxygen ions give electrons to the anode and form oxygen gas.

thank you for these notes