According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 9.
The compounds consisting of hydrogen and carbon only are called hydrocarbons. They are obtained from the major sources of energy like petroleum and coal. Compressed natural gas (CNG) and liquefied petroleum gas (LPG) are the primary sources of energy for the automobile industry, and domestic fuel is obtained from petroleum.
Classification Of Hydrocarbons
Based on the structure, hydrocarbons can be classified into four types:
- Open-chain saturated – Alkanes
- Unsaturated – Unsaturated hydrocarbons are defined as hydrocarbons in which the carbon atoms are bonded to each other with two or three covalent bonds and also have the tendency to obtain more hydrogen atoms. For example, alkynes and alkenes.
- Cyclic – alicyclic
- Aromatic – These hydrocarbons are defined as cyclic hydrocarbons with alternate C-C and C=C, as well as are similar to the benzene ring in characters.
For more information on Hydrocarbons, watch the below videos
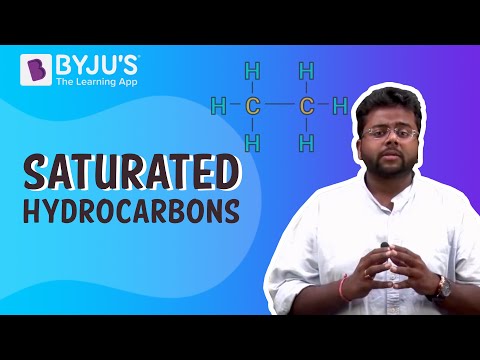

The major reactions of alkanes are oxidation, combustion, aromatization, and free radical substitution. Alkenes and alkynes undertake addition reaction, which is mainly electrophilic addition. Aromatic hydrocarbons mainly undergo electrophilic substitution reactions. They experience additional reactions only under exceptional conditions.
Read More:
| Also Access |
| NCERT Solutions for Class 11 Chemistry Chapter 13 |
| NCERT Exemplar for Class 11 Chemistry Chapter 13 |
Few Important Questions
- Benzene contains three double bonds and is extraordinarily stable. Why?
- How will you convert the hexane into benzene?
- Write chemical equations for the combustion reaction of Toluene and Butane.
Explore more about this chapter and download Hydrocarbons Class 11 Notes PDF register with BYJU’S. Keep visiting us for the latest updates on Class 11 Chemistry Notes.
Other Important Links:
Frequently Asked Questions on CBSE Class 11 Chemistry Notes Chapter 13 Hydrocarbons
Which is the longest hydrocarbon chain?
Palytoxin and maitotoxin are believed to have the longest carbon chains in nature.
What are the types of hydrocarbons?
The four main categories of hydrocarbons: Alkanes, Alkenes, Alkynes and Aromatic hydrocarbons.
What are the uses of cyclic hydrocarbons?
1. Solvent/chemical raw material 2. Manufacture of petroleum products
Comments