According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 9.
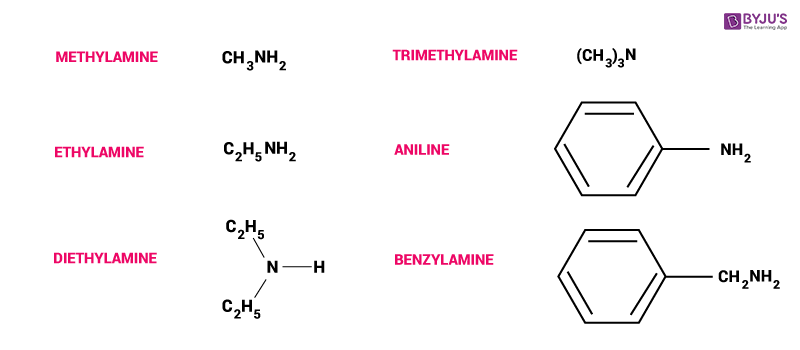
Amines are derived from ammonia acquired by replacing hydrogen atoms in aryl or with alkyl groups. When one hydrogen atom is replaced it gives rise to a primary amine. The structure of secondary amines is given by R2NH or R-NHR′ whereas tertiary amines are characterized by RNR′R′′ or R2NR′ or R3N. If the aryl or alkyl group of tertiary and secondary amines are same they are called simple amines, but if they are attached to different groups they are called mixed amines. Amines have one unshared electron pair on the nitrogen atom, therefore, they behave as Lewis bases. They are formed from halides, amides, nitro compounds or imides.
CBSE Class 12 Chemistry Chapter 13 Amines – Related Links
- Halides
- Amines
- Chemical Reactions of Amines – Acylation and Basicity
- Physical Properties of Amines with Characteristics
- Identification of Primary Amines, Secondary Amines and Tertiary Amines
- Properties and Uses of Amines
- Classification and Nomenclature of Amines
Alkyl Amines
Factors influencing the stability of the substituted ammonium cations in protic polar solvents affecting the basic nature of amines are a combination of electron releasing, H-bonding and steric factors. When compared to ammonia alkyl amines are stronger bases.
Aromatic Amines
The basic character of aromatic amines depends on withdrawing groups and electron releasing. To distinguish and identify primary, secondary, tertiary amines p-Toluenesulphonyl chloride is used. Acylation process controls the reactivity of aromatic amines.
For more information on Amines, watch the below videos

Few Important Questions
- Convert Ethanoic acid to methanamine and Propanoic acid to ethanoic acid
- How to identify primary, secondary, tertiary amines. Writes the reactions involved.
- Is it possible to prepare aromatic primary amines by Gabriel phthalimide synthesis? Why?
- Which of the two has higher boiling point primary amines or tertiary amines? Why?
- Which among the following is a strong base-
Aliphatic amines or aromatic amines? Explain with a valid reason.
- Explain Hofmann’s bromamide reaction.
- Distinguish between Methylamine and dimethylamine with a chemical test.
To discover more about this chapter there are Amines Class 12 CBSE Notes made available with BYJU’S.
Other Important Links:
| Physical Properties Of Amines | Reactions Of Amines |
Frequently Asked Questions on CBSE Class 12 Chemistry Notes Chapter 13 Amines
What are halides?
Halides are chemical compounds that contain halogens. Halides are present in nature with some (salts and acid) being essential to human life.
What are aromatic amines?
An aromatic amine is an organic compound consisting of an aromatic ring attached to an amine.
What is the common name of aniline?
The common name of aniline is aminobenzene.
Comments