According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 11
What Is the Chapter About?
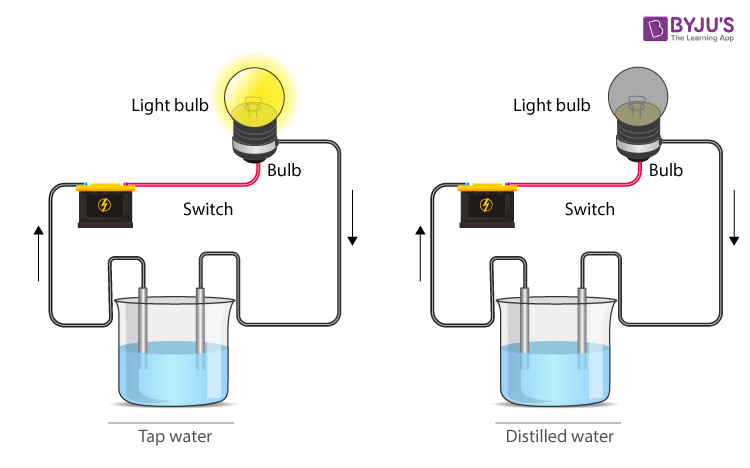
Metals such as copper and aluminium are good conductors of electricity, while materials like rubber and plastics are bad conductors of electricity. In the case of liquids, some liquids are good conductors of electricity, while a few are bad conductors of electricity. The water obtained from sources such as pumps, wells, ponds, and tap are not pure and contain several dissolved salts. Such water is a good conductor of electricity. While distilled water is free of salt and is a bad conductor of electricity.
Chemical effects of electric current
Passage of current through chemical solutions causes chemical reactions to take place.
Chemical effects include:
- formation of gas bubbles at electrodes
- deposition of metals at electrodes
- Changes in solution colour
Electrolysis is the process by which ionic substances are decomposed into simpler substances when an electric current is passed through them.
To know more about Chemical Effects of Electric Current, visit here.
Current Test – Conductors
Conductor
Any material that allows an electric current to pass through it is known as a conductor. Eg: metals like copper
Insulator (Bad Conductors)
Materials that do not allow the free flow of an electric current through them are known as bad conductors or insulators. For example, Rubber and plastic.
To know more about Conductors and Insulators, visit here.
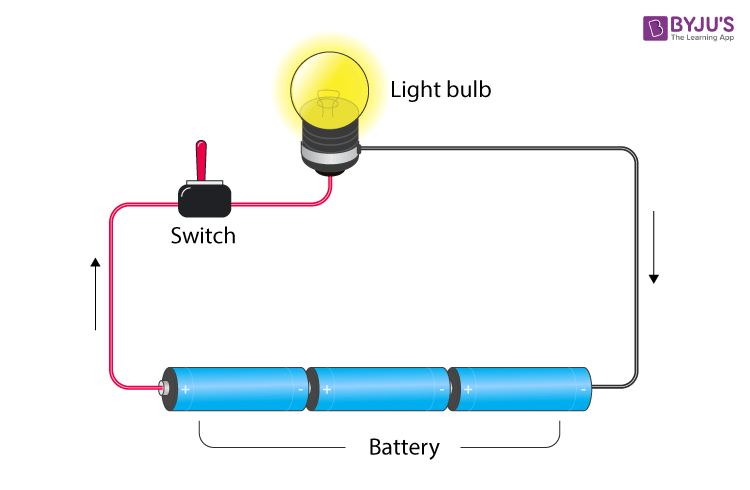
Electric circuit
- A closed-loop path which a current take, is known as an electric circuit.
- When the path of the circuit is closed, the current flows through it, but when there is a break in the path (switch is open) then, the circuit is open and is not conducting.
For more information on the Flow of Current, watch the below video
To know more about Electric Circuit, visit here.
Tester
A tester is a piece of electrical equipment used to check the presence of electric current. It is usually a conductor with a led/bulb to indicate that the current is present in the circuit.
Current – Conducting Liquids
Conducting Liquid
- Liquids conduct electricity too, when there are salts dissolved in the liquid.
- Most liquids that conduct electricity are solutions of acids, bases or salts.
Acids, bases and salts
- Acids and bases are chemical substances that dissociate to form ions when dissolved in a solution. They are a good conductor of electricity because of the presence of the ions.
- Salts, when dissolved in water, also conduct as they release positive/negatively charged ions.
Conduction of electricity in the water
- Distilled water is a bad conductor of electricity because of the absence of dissolved salts and minerals.
- Water starts conducting when acids, bases or salts are dissolved that release ions, which conduct when a potential difference is applied.
To know more about Conduction of Electricity in Liquids, visit here.
Electrodes and electrolyte
- A conductor, when immersed in a solution with its end connected to the terminals of a battery, thereby completing a circuit, is called an electrode. There are usually two electrodes→ cathode (ve) and anode (+ve).
- An electrolyte is a solution in which the electrodes are submerged. They dissociate on the passage of electric current.
- The electrodes, electrolyte and battery together form the electrochemical/electrolytic cell.
Electroplating
Electroplating
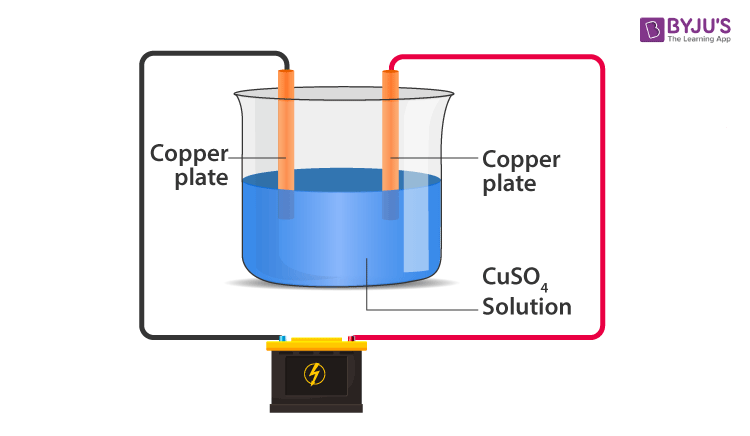
- The process of depositing a layer of desired metal on another material by means of electricity is known as electroplating.
- Example: Using Copper Sulphate solution as electrolyte and copper electrodes. Copper is electroplated on the negative electrode. The Cu in the solution is replenished due to the addition of copper ions from the positive electrode.
Applications of Electroplating
- Coating zinc on the iron to prevent corrosion and rust.
- Coating silver and gold for jewellery.
- Coating tin onto iron for cans as tin is less reactive than iron.
- Chromium coating for car parts, and bath fittings as it has a shiny appearance.
To know more about Electroplating, visit here.
| Also Access |
| NCERT Solutions for Class 8 Science Chapter 14 |
| NCERT Exemplar for Class 8 Science Chapter 14 |
Learn more about the effects of electric current and other related topics, including CBSE class 8 Science notes, at BYJU’S.
Also, Read
| Heating Effect of Electric Current | Electric Current |
Frequently Asked Questions on CBSE Class 8 Science Notes Chapter 14 Chemical Effects of Electric Current
What is Electroplating?
Electroplating is the deposition of a metal coating onto an object by applying a negative charge to it by immersing it in a solution that contains a metal salt.
What are the benefits of Electroplating?
1. Creating a protective barrier 2. Reduces friction 3. Aids in adhesion
How are salts formed?
The substance produced by the reaction of an acid with a base is called a salt.
Comments