The NCERT Exemplar for Class 11 Biology Chapter 9 Biomolecules provides you with the necessary tool required to prepare well for the CBSE Class 11 and entrance examinations, like NEET. This solution is vital for a clear understanding of the topic from the examination point of view.
The NCERT Exemplar for Class 11 Biology Chapter 9 Biomolecules PDF provides answers to the chapter Biomolecules Class 11 NCERT questions, given in the exemplar book, along with Biomolecules essay questions, Biomolecules important questions, Biomolecules question bank, Biomolecules previous years’ questions for the NEET, Biomolecules MCQ GNEET, and tips and tricks.
Download the NCERT Exemplar for Class 11 Biology Chapter 9 Biomolecules PDF
Access Answers to the NCERT Exemplar Solution for Class 11 Biology Chapter 9
MULTIPLE CHOICE QUESTIONS
1. It is said that the elemental composition of living organisms and that of
inanimate objects (like earth’s crust) are similar in the sense that all the
major elements are present in both. Then what would be the difference
between these two groups? Choose the correct answer from among the
following:
a. Living organisms have more gold in them than inanimate objects
b. Living organisms have more water in their body than inanimate
objects
c. Living organisms have more carbon, oxygen and hydrogen per
unit mass than inanimate objects.
d. Living organisms have more calcium in them than inanimate
objects.
Solution:
Option (c) is the answer.
2. Many elements are found in living organisms either free or the form of
compounds. Which of the following is not found in living organisms?
a. Silicon
b. Magnesium
c. Iron
d. SodiumSolution:
Option (a) is the answer.
3. Aminoacids have both an amino group and a carboxyl group in their
structure. Which one of the following is an amino acid?
a. Formic acid
b. Glycerol
c. Glycolic Acid
d. Glycine
Solution:
Option (d) is the answer.
4. An amino acid under certain conditions has both positive and negative
charges simultaneously in the same molecule. Such a form of amino acid
is called
a. Acidic form
b. Basic form
c. Aromatic form
d. Zwitterionic form
Solution:
Option (d) is the answer.
5. Sugars are technically called carbohydrates, referring to the fact that
their formulae are only multiple of C(H2O). Hexoses, therefore, have six
carbons, twelve hydrogens and six oxygen atoms. Glucose is a hexose.
Choose from among the following another hexose.
a. Fructose
b. Erythrose
c. Ribulose
d. Ribose
Solution:
Option (a) is the answer.
6. When you take cells or tissue pieces and grind them with an acid in a
mortar and pestle, all the small biomolecules dissolve in the acid.
Proteins, polysaccharides and nucleic acids are insoluble in mineral acid
and get precipitated. The acid-soluble compounds include amino acids,
nucleosides, small sugars etc. When one adds a phosphate group to a
nucleoside, one gets another acid-soluble biomolecule called
a. Nitrogen base
b. Adenine
c. Sugar phosphate
d. Nucleotide
Solution:
Option (d) is the answer.
7. When we homogenise any tissue in acid the acid-soluble pool
represents
a. Cytoplasm
b. Cell membrane
c. Nucleus
d. Mitochondria
Solution:
Option (a) is the answer.
8. The most abundant component of living organisms is
a. Protein
b. Water
c. Sugar
d. Nucleic acid
Solution:
Option (b) is the answer.
9. A homopolymer has only one type of building block called monomer
repeated ‘n’ number of times. A heteropolymer has more than one type
of monomer. Proteins are heteropolymers usually made ofa. 20 types of monomers
b. 40 types of monomers
c. 30 types of monomers
d. only one type of monomer
Solution:
Option (a) is the answer
10. Proteins perform many physiological functions. For example, some
functions as enzymes. Which of the following represents an additional
function that some proteins discharge?
a. Antibiotics
b. Pigment conferring colour to skin
c. Pigments making colours of flowers
d. Hormones
Solution:
Option (d) is the answer.
11. Glycogen is a homopolymer made of
a. Glucose units
b. Galactose units
c. Ribose units
d. Aminoacids
Solution:
Option (a) is the answer.
12. The number of ‘ends’ in a glycogen molecule would be
a. Equal to the number of branches plus one
b. Equal to the number of branch points
c. One
d. Two, one on the left side and another on the right side
Solution:
Option (d) is the answer.
13. The primary structure of a protein molecule has
a. Two ends
b. One end
c. Three ends
d. No ends
Solution:
Option (a) is the answer.
14. Enzymes are biocatalysts. They catalyse biochemical reactions. In general,
they reduce the activation energy of reactions. Many Physico-chemical
processes are enzyme-mediated. Which of the following reactions is not
enzyme-mediated in the biological system?
a. Dissolving CO2 in water
b. Untwining the two strands of DNA
c. Hydrolysis of sucrose
d. Formation of peptide bond
Solution:
Option (a) is the answer.
VERY SHORT ANSWER TYPE QUESTIONS
1. Medicines are either man-made (i.e., synthetic) or obtained from living
organisms like plants, bacteria, animals etc. and hence the latter are
called natural products. Sometimes natural products are chemically
altered by man to reduce toxicity or side effects. Write against each of
the following whether they were initially obtained as a natural product
or as a synthetic chemical.
a. Penicillin ___________________________
b. Sulfonamide ___________________________
c. Vitamin C ___________________________
d. Growth Hormone ___________________________
Solution:
A. Penicillin- Natural product (obtained from fungus P. notatum)
B. sulphonamide- Synthetic product
C. Vitamin C- Natural product
D. Growth Hormone- Natural product
2. Select an appropriate chemical bond among ester bond, glycosidic bond, peptide bond and hydrogen bond and write against each of the following.
a. Polysaccharide ___________________________
b. Protein ___________________________
c. Fat ___________________________
d. Water ___________________________
Solution:
a. Polysaccharides – Glycosidic bond, formed by elimination of water molecule.
b. Protein- peptide bond, it is the -CO-NH- BOND formed by elimination of water molecules.
c. Fats- ester bond, derived from an acid (organic or inorganic) in which at least one –OH (hydroxyl) group is replaced by an –O–alkyl (alkoxy) group
d. Water- H-bond, formed between the hydrogen of one water molecule and oxygen of the other water molecule.
3. Write the name of anyone amino acid, sugar, nucleotide and fatty acid.
Solution:
Amino acid- Glycine
Sugar- Glucose
Nucleotide- Adenosine
Fatty acid- Oleic acid
4. The reaction given below is catalysed by oxidoreductase between two substrates A and A’, complete the reaction. A reduced + A’ oxidized
Solution:
A[reduced] + A’[Oxidized] → A[oxidized] + A’[reduced]
5. How are prosthetic groups different from co-factors?
Solution:
Cofactors are the no proteinous, may be organic or inorganic constituent of the enzyme. The prosthetic group belongs to organic cofactors, remains tightly bound with apoenzymes.
6. Glycine and Alanine are different with respect to one substituent on the α -carbon. What are the other common substituent groups?
Solution:
-COOH, -NH2, and –H are the common substituents.
7. Starch, Cellulose, Glycogen, Chitin are polysaccharides found among the following. Choose the one appropriate and write against each.
Cotton fibre __________________________
Exoskeleton of cockroach __________________________
Liver __________________________
Peeled potato __________________________
Solution:
Cotton fibre- cellulose [more than 90%].
The exoskeleton of cockroach- chitin
Liver- glycogen
Peeled potato- starch
SHORT ANSWER TYPE QUESTIONS
1. Enzymes are proteins. Proteins are long chains of amino acids linked to
each other by peptide bonds. Aminoacids have many functional groups
in their structure. These functional groups are, many of them at least,
ionisable. As they are weak acids and bases in chemical nature, this
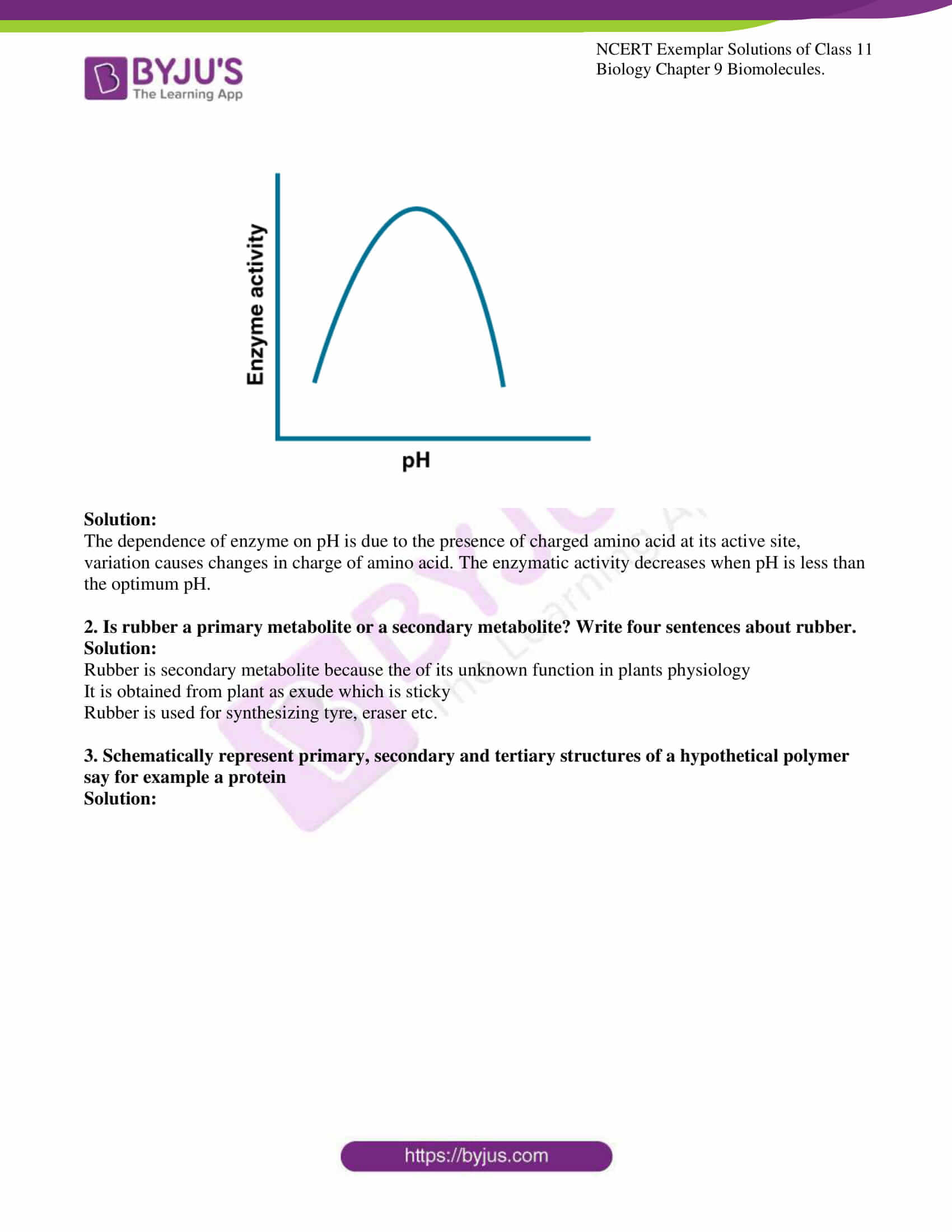
ionization is influenced by the pH of the solution. For many enzymes, activity
is influenced by the surrounding pH. This is depicted in the curve below,
explain briefly.
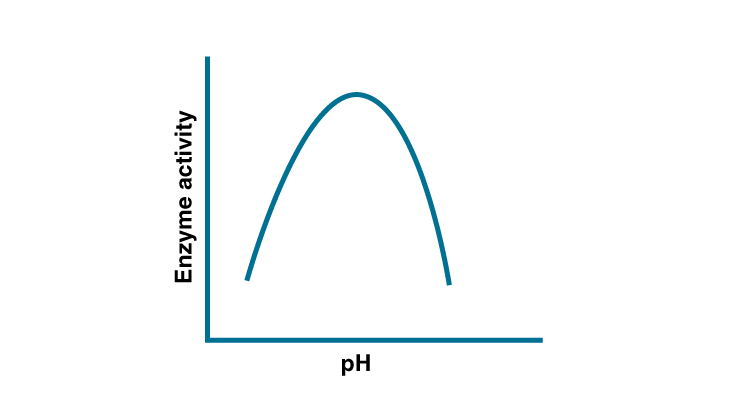
Solution:
The dependence of enzyme on pH is due to the presence of charged amino acid at its active site, variation causes changes in charge of amino acid. The enzymatic activity decreases when pH is less than the optimum pH.
2. Is rubber a primary metabolite or a secondary metabolite? Write four sentences about rubber.
Solution:
Rubber is secondary metabolite because the of its unknown function in plants physiology
It is obtained from plant as exude which is sticky
Rubber is used for synthesizing tyre, eraser etc.
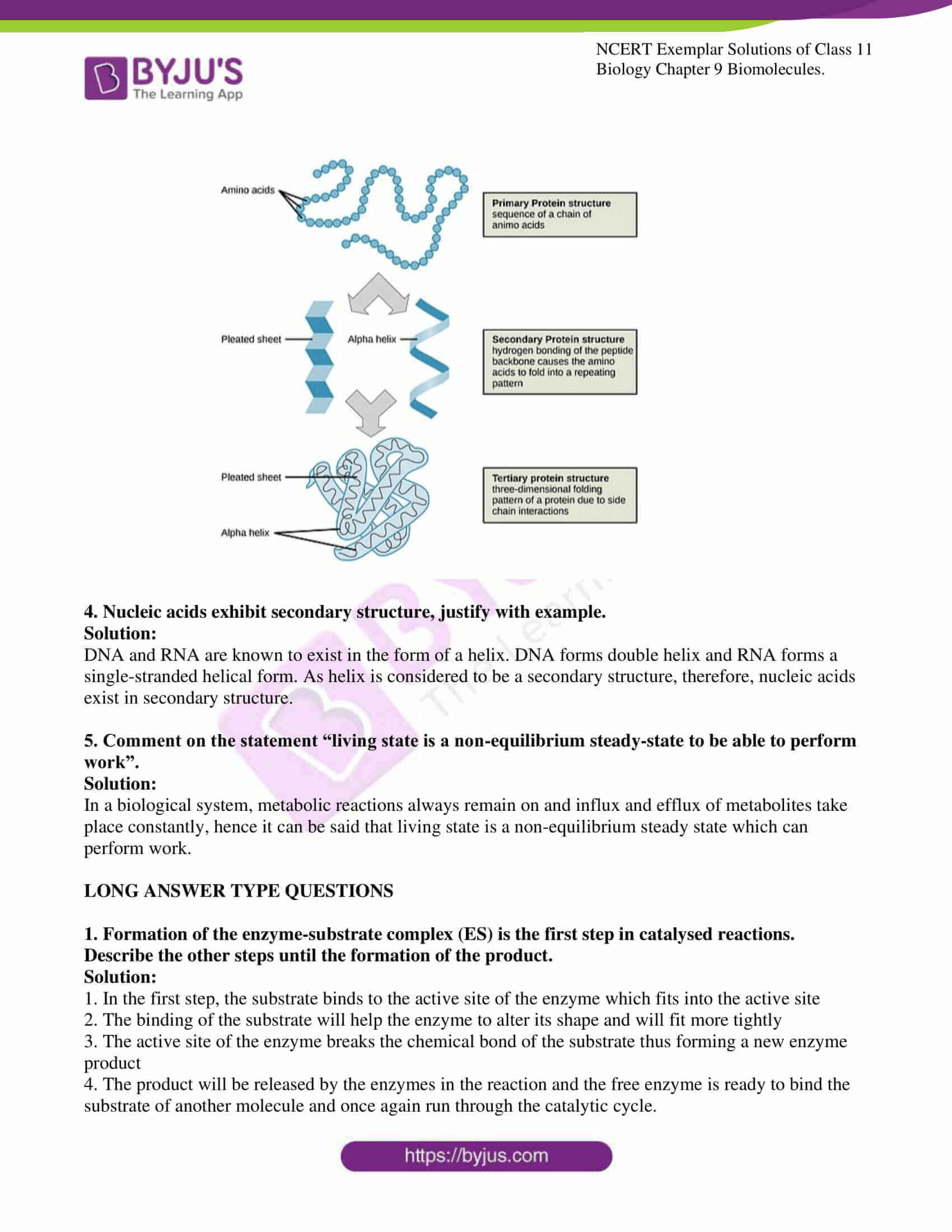
3. Schematically represent primary, secondary and tertiary structures of a hypothetical polymer say for example a protein
Solution:
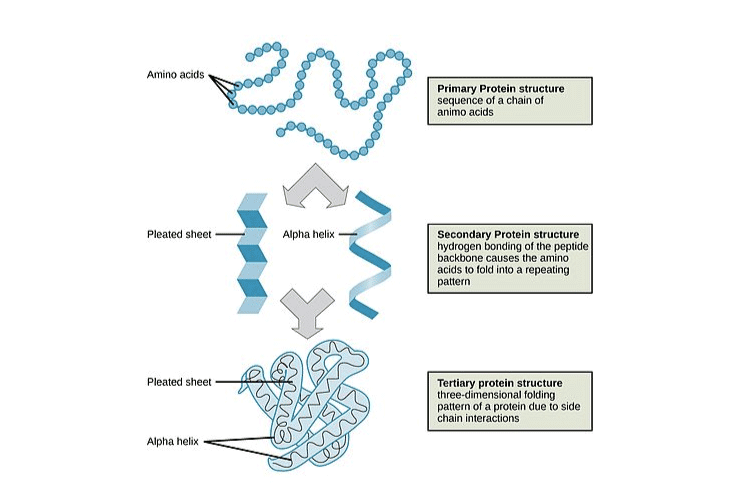
4. Nucleic acids exhibit secondary structure, justify with example.
Solution:
DNA and RNA are known to exist in the form of a helix. DNA forms double helix and RNA forms a single-stranded helical form. As helix is considered to be a secondary structure, therefore, nucleic acids exist in secondary structure.
5. Comment on the statement “living state is a non-equilibrium steady-state to be able to perform work”.
Solution:
In a biological system, metabolic reactions always remain on and influx and efflux of metabolites take place constantly, hence it can be said that living state is a non-equilibrium steady state which can perform work.
LONG ANSWER TYPE QUESTIONS
1. Formation of the enzyme-substrate complex (ES) is the first step in catalysed reactions. Describe the other steps until the formation of the product.
Solution:
1. In the first step, the substrate binds to the active site of the enzyme which fits into the active site
2. The binding of the substrate will help the enzyme to alter its shape and will fit more tightly
3. The active site of the enzyme breaks the chemical bond of the substrate thus forming a new enzyme product
4. The product will be released by the enzymes in the reaction and the free enzyme is ready to bind the substrate of another molecule and once again run through the catalytic cycle.
2. What are the different classes of enzymes? Explain any two with the type of reaction they catalyse.
Solution:
There are different classes of enzymes. They are Oxidoreductase, Transferase, Hydrolase, Lysates, Isomerase, ligases.
a) Oxidoreductase:
These are the enzymes which help in simultaneous oxidation and reduction of two substrates.
S reduced + S’reduced → S oxidized + S’ oxidized
b) Hydrolases:
These enzymes facilitate the hydrolysis of a molecule.
Sucrose → Glucose + Fructose
3. Nucleic acids exhibit secondary structure. Describe through Watson Crick Model.
Solution:
DNA is made up of two polypeptide chains, arranged in a double helix. The backbone is of sugar-phosphate while the nitrogenous bases are present on the inner side. These nitrogenous bases pairs to each other by H bonds. This H bond confers for the stability of helix.
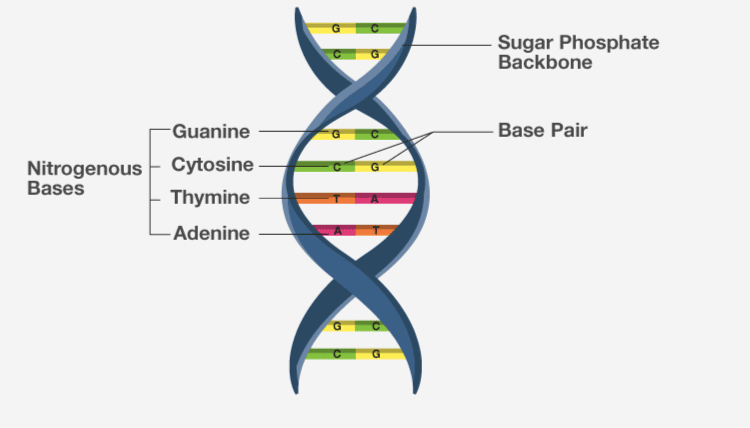
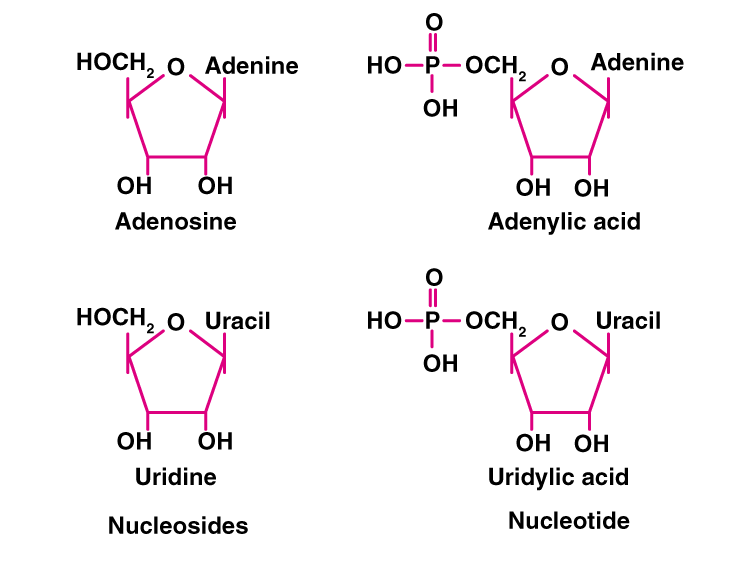
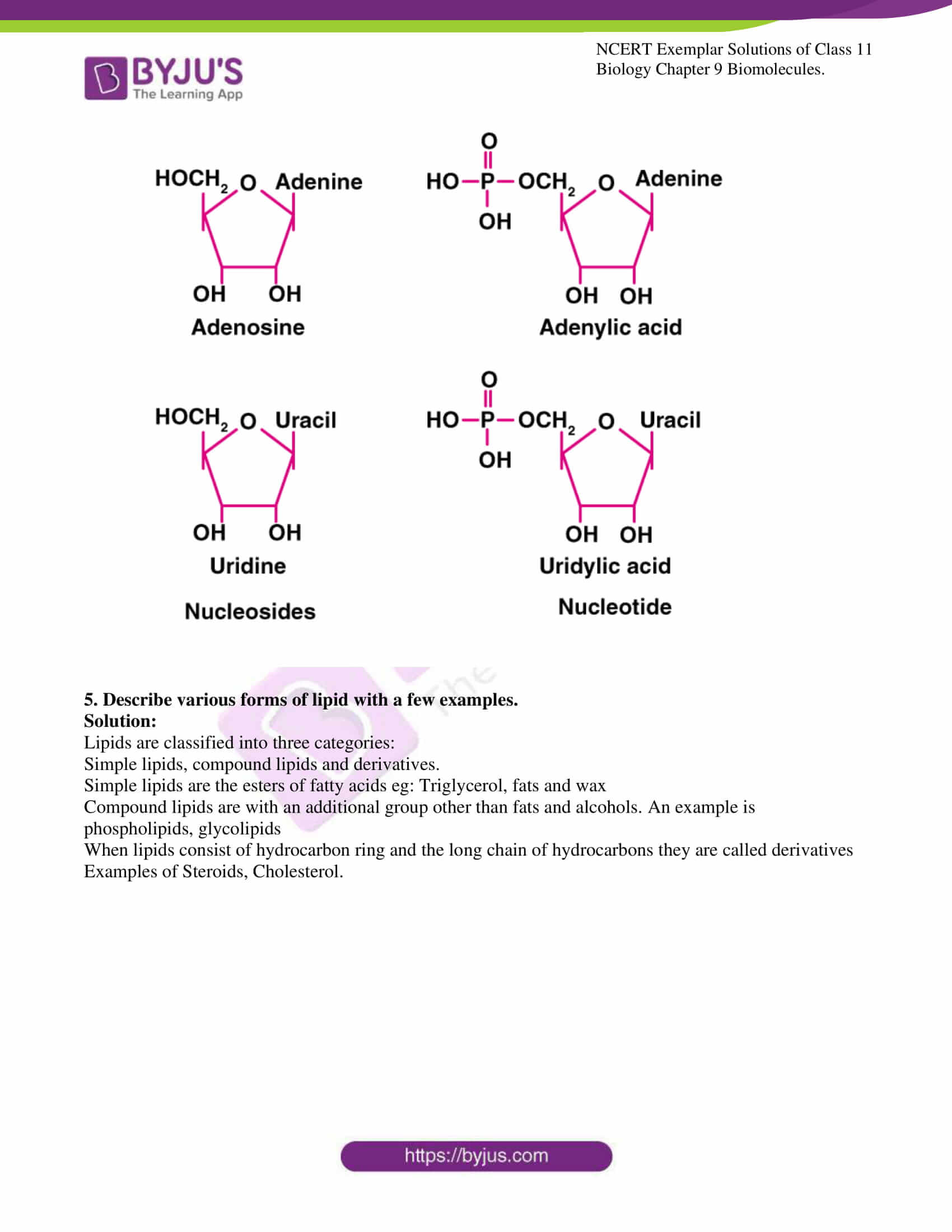
4. What is the difference between a nucleotide and nucleoside? Give two examples of each with their structure.
Solution:
Nucleotides are the monomers of nucleic acid and are formed of nucleoside and phosphate group. Whereas nucleosides are the constituent of nucleotides. Adenosine, guanosine, thymidine, uridine and cytidine are nucleosides. Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cacodylic acid are nucleotides.
5. Describe various forms of lipid with a few examples.
Solution:
Lipids are classified into three categories:
Simple lipids, compound lipids and derivatives.
Simple lipids are the esters of fatty acids eg: Triglycerol, fats and wax
Compound lipids are with an additional group other than fats and alcohols. An example is phospholipids, glycolipids
When lipids consist of hydrocarbon ring and the long chain of hydrocarbons they are called derivatives
Examples of Steroids, Cholesterol.
Biomolecules can be defined as molecules present in different organisms, which are responsible for a whole host of biological processes. Different processes, such as cell division and development, are run by biomolecules. Some types of biomolecules are as follows.
- Metabolites
- Hormones
- Neurotransmitters
- Vitamins
- Lipids and Fatty acids
| Also Access |
| NCERT Solutions for Class 11 Biology Chapter 9 |
| CBSE Notes for Class 11 Biology Chapter 9 |
Important Topics of Chapter 9 Biomolecules
-
- Analysing Chemical Composition?
- Primary and Secondary Metabolites
- Bio-macromolecules
- Proteins
- Polysaccharides
- Nucleic Acids
- Structure of Proteins
- Nature of Bond Linking Monomers in a Polymer
- Dynamic State of Body Constituents – Concept of Metabolism
- Metabolic Basis for Living
- The Living State
- Enzymes
BYJU’S delivers highly innovative learning content for CBSE students. BYJU’S video and animation lessons, worksheets, exercises, notes, book, and tips and tricks assist students in understanding the concepts and clearing their doubts.






Comments